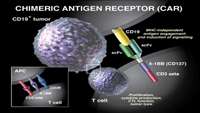
Novartis announces first CAR-T cell therapy BLA for pediatric and young adult patients with r/r B-cell ALL granted FDA Priority Review
Novartis announced today that the US Food and Drug Administration (FDA) has accepted the companys Biologics License Application (BLA) filing and granted priority review for CTL019, an investigational chimeric antigen receptor T cell (CAR-T) therapy, in relapsed and refractory (r/r) pediatric and young adult patients with B-cell acute lymphoblastic leukemia (ALL). This is the first BLA submission by Novartis for a CAR-T. The priority review designation is expected to shorten the anticipated review time by the FDA.
Sickle Cell Anemia Patient Cured by Gene Therapy
In a world first, a teenager with sickle cell disease achieved complete remission after an experimental gene therapy at Necker Childrens Hospital in Paris, researchers say.
Life expectancy forecast to exceed 90 years in coming decades
Study shows significant increase in lifespan, with South Korea top of league table and other countries not far behind
Stem cell treatment could be a proven therapy for diabetes, autism
Stem cell therapy has turned out to be a ray of hope for most patients who are trying to find a cure for incurable diseases.
Stem cell therapy has turned out to be a ray of hope for most patients who are trying to find a cure for incurable diseases. Researchers and experts believe India has been at the top in the development of stem cell treatment followed by several other countries like China and Japan. However, due to lack of awareness, most people dont think of stem cell therapy as an option for treating most of the incurable diseases.
Worlds first baby born from new procedure using DNA of three people
Experts welcome news of successful mitochondrial transfer but caution against operating in countries beyond regulations
Goodbye Arthritis: Stem Cell Therapy Could Revolutionize Hip Replacement
Arthritis-fighting stem cells may eliminate the need for joint replacement surgeries, scientists said.
The breakthrough gene-editing technology is moving forward
According to Time.com website, An advisory panel at the National Institutes of Health (NIH) has approved a proposal to use the gene-editing technology CRISPR on human cells. It’s the first trial involving humans to be approved in the U.S.
Second baby gets Cellectis "designer" cells to clear leukaemia
Second baby gets Cellectis "designer" cells to clear leukaemia
Hairy Skin from Stem Cells
Scientists just got one step closer to growing functional skin in vitro