Global CAR T Cell Therapy Market to Reach US$ 8.5 Billion by 2028
The Global Car T Cell Therapy Market is expected to be valued at US$ 8.5 Billion by 2028, as analyzed by Coherent Market Insights. Increasing demand for genetically engineered treatment approach for high success rate in cancer treatment along with growing cancer incidence rate are key factors driving demand for CAR T cell therapy products worldwide.
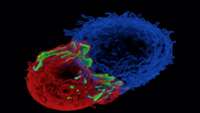
Engineered T cells for all
Gene-engineered autologous T cells with chimeric antigen receptors (CARs) against CD19 (CART19 cells) have been effective against patients with CD19+ B cell malignancies.
UCLA Launches First-of-Its-Kind Stem Cell Trial to Combat Cancer
Scientists at UCLA have launched the first genetically engineered blood stem cell clinical trial to fight cancer thanks to $20 million in voter-approved funds earmarked for stem cell research, university officials announced Monday.
Gene therapy restores auditory and vestibular function in a mouse mode
Because there are currently no biological treatments for hearing loss, we sought to advance gene therapy approaches to treat genetic deafness.
6 Companies Developing Cell Therapy Treatments for Diabetes6 Companies Developing Cell Therapy Treatments for Diabetes
With an aging population, obesity on the rise, and sedentary lifestyles becoming increasingly common, diabetes has grown into a major health concern in the U.S. and worldwide. According to the Center for Disease Control (CDC), 29.1 million people or 9.3% of the U.S. population has diabetes, including an estimated 8.1 million who are undiagnosed. According to the American Diabetes Association, the 2012 cost of this disease in the U.S. was “$245 billion per annum or approximately $500,000 every minute.”
A “Fourth-Generation” DNA Base Editor Could Replace CRISPR
The latest news in genetic science has been dominated by the CRISPR/Cas9 technique over the past five years. But a new “fourth-generation” DNA base editor could see CRISPR dethroned, according to a recent study published in Science Advances.
CD19-Specific CAR T-Cell Therapy Effective in Ibrutinib-Refractory CLL
Anti-CD19 chimeric antigen receptor (CAR)-modified T-cell therapy was highly effective in patients with high-risk chronic lymphocytic leukemia (CLL) who had previously failed treatment with ibrutinib, according to the results of a study published in the Journal of Clinical Oncology.
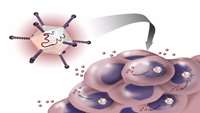
Immatics Initiates Personalized Adoptive Cellular Therapy in Patients with Relapsed And/Or Refractory Solid Cancers Using Its Pioneering Target Warehouse
Immatics, a leading company in the field of cancer immunotherapy, today announced that it has initiated enrollment of patients into a phase I trial of its first adoptive cellular therapy (ACT) IMA101, using its proprietary ACTolog® approach. The IMA101 phase I trial is the first industry-sponsored trial using products consisting of autologous cytotoxic T lymphocytes targeting defined tumor antigens using Immatics’ novel and proprietary target warehouse. This single-center study is now open for enrollment at The University of Texas MD Anderson Cancer Center in Houston, Texas.
Genome editing for scalable production of alloantigen‐free lentiviral vectors for in vivo gene therapy
Lentiviral vectors (LV) are powerful and versatile vehicles for gene therapy. However, their complex biological composition challenges large‐scale manufacturing and raises concerns for in vivo applications, because particle components and contaminants may trigger immune responses
New electric mesh device gives the heart an electromechanical hug
The study, published in the June 22, 2016 issue of Science Translational Medicine, points to a potential new way of improving heart function and treating dangerous arrhythmias by compensating for damaged cardiac muscle and enabling living heart muscle to work more efficiently.