Engineered T cells for all
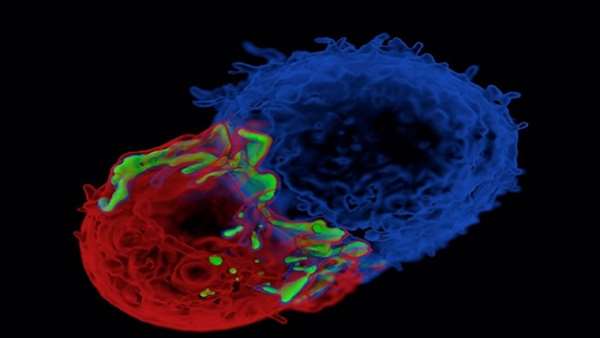
Gene-engineered autologous T cells with chimeric antigen receptors (CARs) against CD19 (CART19 cells) have been effective against patients with CD19+ B cell malignancies.
Gene-engineered autologous T cells with chimeric antigen receptors (CARs) against CD19 (CART19 cells) have been effective against patients with CD19+ B cell malignancies. However, the personalized process of T cell selection from patients, and engineering them to express CAR‑CD19 is laborious and sometimes impossible in patients with severe lymphopenia. So, the field has been looking to recent advances in gene engineering technologies and to the possibility of generating universal engineered T cells from donors.
Qasim et al. used transcription activator-like effector nucleases (TALENs) to engineer human leukocyte antigen (HLA)- mismatched donor T cells to produce universal CART19 cells. The donor T cells were transduced with lentivirus to express CAR‑CD19 and they were engineered by electroporation with TALENs to ablate CD52 and T cell receptor (TCR)-α constant region (TRAC). CD52 was disrupted so the cells could evade and survive alemtuzumab (which targets CD52), which is used as a conditioning therapy before therapeutic stem cell transplantation (SCT). TRAC was ablated to prevent TCRα–TCRβ expression so as to minimize the risk of graft-versus-host disease (GVHD), which is a concern when using HLA-unmatched cell transplants. These universal CART19 (UCART19) cells were also engineered to express a CD20 epitope so they could be cleared by treatment with rituximab in situations of adverse effects.
The authors then treated two infants with relapsed refractory CD19+CD52– B cell acute lymphocytic leukaemia (B-ALL). Infant 1 received a single dose and suffered no immediate adverse toxicities and was found to be in remission within 4 weeks. The UCART19 cells reached 27% frequency in bone marrow although blood cell counts were low and by 9 weeks there was evidence of skin GVHD from residual TCRα–TCRβ+ cells, which was managed with steroid treatment. Rituximab was used to clear the UCART19 cells and various conditioning agents were administered to prepare for a second SCT. The infant remains in molecular remission 18 months later. Infant 2 had mixed lineage leukaemia (MLL)- rearranged B-ALL. On relapse, this patient received UCART19 cells and was also found to be in remission by 1 month. Then, the patient received an allogeneic SCT after rituximab treatment and additional conditioning therapy. There was no evidence of GVHD and the patient is in molecular remission 12 months after therapy.
This report provides the first example of using universal engineered CAR T cells from HLA-mismatched donors, suggesting that far more patients — including those with the severest disease — might benefit from this therapy.
Nature Reviews Cancer 17, 206–207. (2017): doi:10.1038/nrc.2017.19



ارسال به دوستان