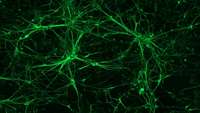
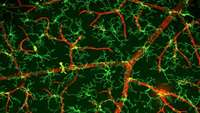
Zebrafish study reveals regenerative processes by neural stem cells in the brain
Waseda University researchers recently elucidated the regenerative processes of neural stem cells using a stab injury model in the optic tectum of adult zebrafish. The study could contribute to the treatment of human central nervous system (CNS) injuries.
Scientists Have Discovered a New Stem Cell That Could Heal Brain Damage
A newly discovered type of stem cell could help brains repair themselves from injury or even debilitating diseases like Alzheimers, according to the latest research.
Stem Cell Assay Market Worth 1,978.7 Million USD by 2023
According to a new market research report "Stem Cell Assay Market by Type (Viability, Purification, Identification), Cell Type (Mesenchymal, iPSCs, HSCs, hESCs), Product & Service (Instruments, Kits), Application (Regenerative Medicine, Clinical Research), End User - Global Forecast to 2023", published by MarketsandMarkets™, the market is expected to reach USD 1,978.7 Million by 2023 from USD 791.9 Million in 2018, at a CAGR of 20.1%.
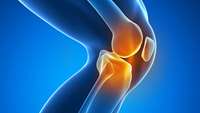
Stem cells: A natural alternative to surgery
Stem cell therapy has produced impressive results in treating musculoskeletal conditions such as osteoarthritis and painful sprains and tears of muscles, ligaments and tendons. But harvesting stem cells from the body can be invasive and painful – is there an alternative? Yes, according to The Knee Institute and Regenerative Therapy, a clinic in West Bloomfield that uses only stem cells from human umbilical cords for the best results.
13-Year-Old Boy Is First Person in US to Receive Newly Approved Gene Therapy for Blindness
On Tuesday, a 13-year-old boy from New Jersey was at the center of medical history as he became the first person in the US to receive an FDA-approved gene therapy for an inherited disease. The event marks the beginning of a new era of medicine, one in which devastating genetic conditions that we are born with can be simply edited out of our DNA with the help of modern biomedical technologies.
The Leader picks “Support for Iranian Products” as New Year’s motto:
All must work to realize this year’s motto
In a message marking the arrival of the Solar calendar year of 1397 (beginning March 21, 2018), Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei congratulated all Iranian compatriots, particularly the respectable families of martyrs and the war-wounded as well as promising youths and teenagers, who are the driving force behind the country’s scientific movement.
President Hassan Rouhani:
Great Steps Have Been Taken in Various Fields During Last Year
President Rouhani described year 1396 as the year of success for the great Iranian nation in various fields despite the wish of the enemies and said: “The unity among the Iranian nation has surprised all enemies and they admitted to the greatness of our people”.
Researchers move one step closer towards functioning kidney tissue from stem cells
Researchers from the Murdoch Childrens Research Institute (MCRI), University of Melbourne and Leiden University Medical Centre (LUMC) in The Netherlands have made an important step towards making human kidneys from stem cells that they one day hope can be used to treat kidney disease.
Chinese scientists decipher origins of repopulated microglia in brain and retina
The regenerative capability of the central nervous system (CNS) is largely limited due to its intrinsic properties and external environment. Traditional thinking holds that once the brain is injured, it is impossible to repair and restore the tissue to normal. However, this notion has been challenged by a recent study.
First allogeneic stem cell therapy to receive central marketing authorization approval in Europe
TiGenix NV (Euronext Brussels and NASDAQ: TIG) ("TiGenix") and Takeda Pharmaceutical Company Limited (TSE: 4502) ("Takeda") today announced that the European Commission (EC) has approved Alofisel (darvadstrocel), previously Cx601, for the treatment of complex perianal fistulas in adult patients with nonactive/mildly active luminal Crohns disease, when fistulas have shown an inadequate response to at least one conventional or biologic therapy. Alofisel should be used after conditioning of fistula.[2] This marks the first allogeneic stem cell therapy to receive central marketing authorization (MA) approval in Europe.