Chinese scientists decipher origins of repopulated microglia in brain and retina
The regenerative capability of the central nervous system (CNS) is largely limited due to its intrinsic properties and external environment. Traditional thinking holds that once the brain is injured, it is impossible to repair and restore the tissue to normal. However, this notion has been challenged by a recent study.
The regenerative capability of the central nervous system (CNS) is largely limited due to its intrinsic properties and external environment. Traditional thinking holds that once the brain is injured, it is impossible to repair and restore the tissue to normal. However, this notion has been challenged by a recent study.
Microglia are long-lived myeloid cells in the central nervous system (CNS). Elmore and Green et al. reported newly discovered microglial progenitor cells in Neuron in 2014. This finding showed for the first time microglial progenitors in the adult brain, providing potential insights for regenerative medicine. However, the origin of repopulated microglia is still hotly debated, and the source of repopulated microglia remains highly controversial.
Recently, a research paper entitled "Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion" was published in Nature Neuroscience by the laboratory of Bo Peng at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences. In this paper, PENG and his colleagues successfully deciphered the origin of repopulated microglia in the brain by a series of fate mapping approaches.
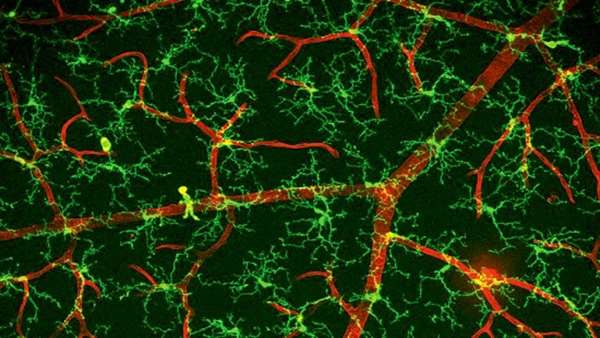
Researchers first excluded the blood origin of repopulated microglia via parabiosis, a surgical approach generating chimeric mice with exchanged blood cells (Figure 1a). They then demonstrated that repopulated microglia were NOT differentiated from Nestin-positive progenitor cells (Figure 1b).
They also proved that astrocytes, oligodendrocyte precursor cells (OPCs) and neurons were not the precursor cells of repopulated microglia (Figure 1c). In contrast, ALL repopulated microglia in the brain were derived from the proliferation of the few microglia surviving after pharmacological ablation (<1%), and the dividing microglia transiently expressed Nestin (Figure 1d).
The results provided solid evidence that repopulated microglia were solely derived from residual microglia rather than de novo progenitors, indicating the absence of microglial progenitor cells in the adult brain. In addition, through RNA sequencing, researchers found that the repopulated microglia may share similar functions as the resident microglia in homeostatic and diseased brains.
Moreover, the retina is an important part of the CNS. The capacity for cell regeneration is even more limited in the adult mammalian retina than in the brain. Although a few cells are sporadically regenerated in the retina, no massive cell regeneration had previously been observed.
In a paper entitled "Dual extra-retinal origins of microglia in the model of retinal microglia repopulation" published in Cell Discovery, PENG and his colleagues found that inhibition of CSF1R eliminated all resident microglia in the retina (100%) (Figure 2), in contrast with 99% of the brain.
If the retina shares a similar mechanism for microglia repopulation as that of the brain (Figure 1), newborn microglia will not emerge and repopulate the retina since there are no surviving microglia after depletion (Figure 2). However, the authors found that new microglia emerged and rapidly repopulated the whole retina after removal of CSF1R inhibition (Figure 2). This is the first time robust and massive cell regeneration has been observed in the adult mammalian retina. And the origin of the retinal microglia is a mystery.
The authors then investigated the origin of repopulated retinal microglia and found that repopulated retinal microglia have two populations of distinct origin: the majority, which are center-emerging microglia; and the minority, which are periphery-emerging microglia.Center-emerging microglia were solely derived from residual microglia in the optic nerve, whereas periphery-emerging microglia were derived from macrophages in the ciliary body/iris (Figure 3).
Through the microglial repopulation model, the authors for the first time observed robust and massive cell regeneration in the adult mammalian retina. Furthermore, they first identified the extra-retinal origin of microglia in the adult retina, shedding new light on the origins and maintenance of microglia in the retina.
These studies were mainly conducted by the laboratory of Bo Peng in collaboration with Dr. Yanxia Rao at the University of Hong Kong and Prof. Ti-Fei Yuan at Shanghai Jiao Tong University. Dr. Yubin Huang and Dr. Zhen Xu are the co-first authors of these two studies.
Reference: http://dx.doi.org/10.1038/s41593-018-0090-8



ارسال به دوستان