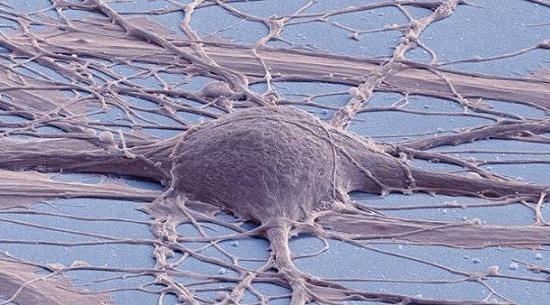
creation spinal cord neural stem cells (NSCs) from human pluripotent stem cells (hPSCs)
The achievement, described in the August 6 online issue of Nature Methods, advances not only basic research like biomedical applications of in vitro disease modeling, but may constitute an improved, clinically translatable cell source for replacement strategies in spinal cord injuries and disorders.
The achievement, described in the August 6 online issue of Nature Methods, advances not only basic research like biomedical applications of in vitro disease modeling, but may constitute an improved, clinically translatable cell source for replacement strategies in spinal cord injuries and disorders.
In recent years, much work has been done exploring the potential of using hPSC-derived stem cells to create new spinal cord cells needed to repair damaged or diseased spinal cords. Progress has been steady but slow and limited.
In their new paper, first author and postdoctoral scholar Hiromi Kumamaru, MD, PhD, and senior author Mark Tuszynski, MD, PhD, professor of neuroscience and director of the UC San Diego Translational Neuroscience Institute, and colleagues describe creating a cell line that appears to significantly advance the cause.
After grafting cultured hPSC-derived NSCs into injured spinal cords of rats, they noted that the grafts were rich in excitatory neurons, extended large numbers of axons over long distances, innervated their target structures and enabled robust corticospinal regeneration.
"We established a scalable source of human spinal cord NSCs that includes all spinal cord neuronal progenitor cell types," said Kumamaru. "In grafts, these cells could be found throughout the spinal cord, dorsal to ventral. They promoted regeneration after spinal cord injury in adult rats, including corticospinal axons, which are extremely important in human voluntary motor function. In rats, they supported functional recovery."
Tuszynski said that, although more work needs to be done, these newly generated cells will constitute source cells for advancement to human clinical trials on a time frame of three to five years. It still needs to be determined that the cells are safe over long time periods in rodent and non-human primate studies, and that their efficacy can be replicated.
He noted that the work presents potential benefits beyond spinal cord injury therapies since the NSCs can be used in modeling and drug screening for disorders that also involve spinal cord dysfunction, such as amyotrophic lateral sclerosis, progressive muscular atrophy, hereditary spastic paraplegia and spinocerebellar ataxia, a group of genetic disorders characterized by progressive discoordination of gait, hands and eye movement.
Co-authors include: Ken Kadoya, Andrew F. Adler, Yoshio Takashima, and Lori Graham, all at UC San Diego, and Giovanni Coppola, UCLA.
Reference : https://www.nature.com/articles/s41592-018-0074-3




ارسال به دوستان