The unique antitumor characteristics of γδ T cells are being put to the test in a first-of-its-kind clinical study that may pave the way to a new class of immunotherapy. In the coming weeks, TC BioPharm will begin administering its proprietary formulation of donor-derived γδ T cells to patients with acute myeloid leukemia (AML) as part of a late-stage, pivotal trial designed to evaluate whether these ‘unconventional’ lymphocytes can specifically recognize cancers and rally an immune response against them — even without the addition of chimeric antigen receptors (CARs) or other types of genetic manipulation.
The trial should provide “proof of principle of whether it will feasible to use these off-the-shelf products,” says hematologist Emma Nicholson, a site investigator at the Royal Marsden Hospital in Sutton, UK. “There’s a lot of interest in whether γδ T cells have got antitumor activity.” Today, more than a dozen different companies are pushing ahead with γδ T cell–based therapies of their own — and so, if TC BioPharm succeeds with its therapy, many others could soon follow.
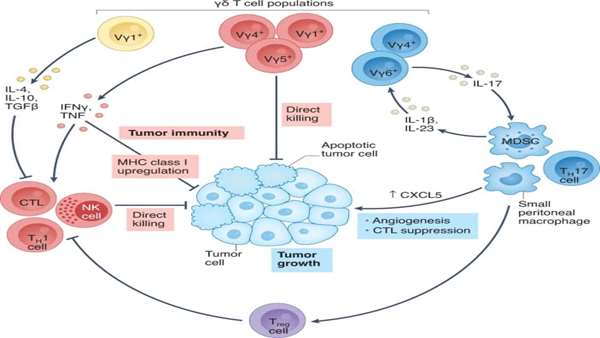
That therapy, termed OmnImmune, takes advantage of a peculiarity of γδ T cells, an immune population that combines both innate and adaptive features. Unlike most T lymphocytes, these cells carry T cell receptors
(TCRs) composed of γ and δ chains, which recognize antigens without depending on major histocompatibility complex (MHC) molecules. This characteristic sets the approach apart from other T cell immunotherapeutic strategies built around more classic αβ cells, which are restricted to recognize MHC-bound peptides that go through the usual antigen processing and presentation pathways. And it allows the creation of ‘universal’ γδ T cells treatments — sourced from the blood of healthy individuals, then expanded, activated and purified — without the complicating risk of graft-versus-host-disease that necessitates further genetic engineering to make allogeneic αβ T cell therapies feasible.
“This is a population of cells that can have strong antileukemia activity,” says Alice Bertaina, a pediatric stem cell transplant specialist at Stanford University, who is not involved in the TC BioPharm trial. “The γδ T cells, even when not engineered with CARs — so, without specific targets — have the potential of mediating much more graft-versus-tumor effects than αβ T cells.”
Some companies are engineering γδ T cells nonetheless, equipping their therapies with CARs for enhanced tumor targeting and killing efficiency (Table 1). Adicet Bio, for example, incorporated a CD20-directed CAR into its lead
candidate, ADI-001, which is designed to combat B cell lymphomas. According to clinical data announced in December 2021, the therapy yielded several complete and near-complete responses in the first handful of recipients. Updated results will be presented at the 2022 meeting of the American Society of Clinical Oncology.
But TC BioPharm, like a handful of other firms, is focused first on deploying ‘naked’, unmodified cells — the goal being to harness the natural properties of γδ T cells to detect and destroy malignant cells while sparing normal, healthy tissues. In addition to OmnImmune — which is being trialed in a pre-transplant setting for patients with AML who did not respond well to first-line chemotherapy — there is INB-100, another unmodified γδ T cell candidate. INB-100 seems to help prevent relapse when used as post-transplant treatment for leukemia. Scientists at IN8bio, the company behind INB-100, reported phase 1 trial findings in March.



ارسال به دوستان