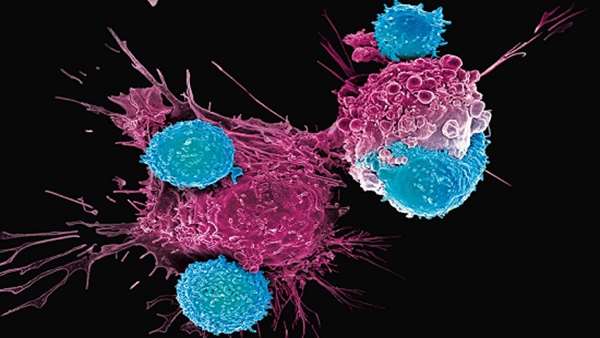
US FDA approves Cellectis’ third ‘off-the-shelf’ CAR T-cell candidate for clinical trials
The FDA approved an investigational new drug application from Cellectis for a Phase 1 clinical trial of UCART22, its third gene-edited CAR-T cell candidate.
The FDA approved an investigational new drug application from Cellectis for a Phase 1 clinical trial of UCART22, its third gene-edited CAR-T cell candidate.
The therapy uses engineered cells from healthy donors rather than a patient’s own cells. The approval comes the month after the French company submitted the IND, which aims to treat adult patients with B-cell acute lymphoblastic leukemia.
Cellectis plans to initiate a Phase I clinical study later this year at the University of Texas MD Anderson Cancer Center.



ارسال به دوستان