Removable implant may control type 1 diabetes
For the more than 1 million Americans who live with type 1 diabetes, daily insulin injections are literally a matter of life and death. And while there is no cure, a Cornell-led research team has developed a device that could revolutionize management of the disease.
For the more than 1 million Americans who live with type 1 diabetes, daily insulin injections are literally a matter of life and death. And while there is no cure, a Cornell-led research team has developed a device that could revolutionize management of the disease.
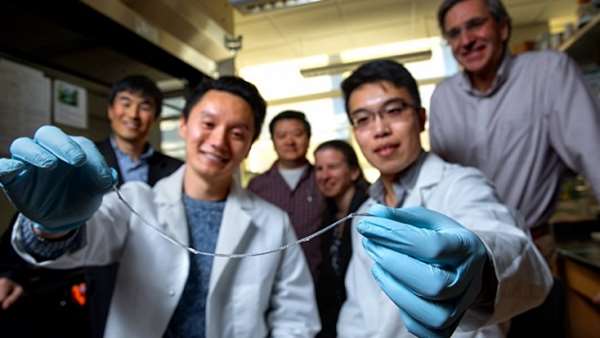
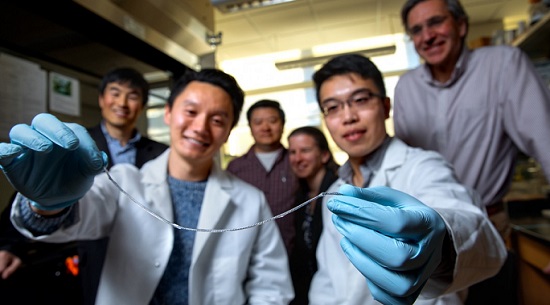
In Type 1 diabetes, insulin-producing pancreatic cell clusters (islets) are destroyed by the body’s immune system. The research group, led by assistant professor Minglin Ma from the Department of Biological and Environmental Engineering in the College of Agriculture and Life Sciences, has devised an ingenious method for implanting hundreds of thousands of islet cells into a patient. They are protected by a thin hydrogel coating and, more importantly, the coated cells are attached to a polymer thread and can be removed or replaced easily when they have outlived their usefulness.
Doctoral students Duo An and Alan Chiu are co-lead authors of the group’s paper, “Designing a Retrievable and Scalable Cell Encapsulation Device for Potential Treatment of Type 1 Diabetes,” published Dec. 25 in Proceedings of the National Academy of Sciences.
An example of the “radical collaboration” concept that is a hallmark of Cornell research, this work also featured key contributions from: Dr. James Flanders from the College of Veterinary Medicine; professor Jintu Fan from the Department of Fiber Science & Apparel Design in the College of Human Ecology; and assistant professor Meredith Silberstein from the Department of Mechanical and Aerospace Engineering in the College of Engineering.
Transplantation of stem cell-derived, insulin-producing islet cells is an alternative to insulin therapy, but that requires long-term immunosuppressive drug administration. One well-researched approach to avoid the immune system’s response is to coat and protect the cells in tiny hydrogel capsules, hundreds of microns in diameter. However, these capsules cannot be taken out of the body easily, since they’re not connected to each other, and there are hundreds of thousands of them.
And the ability to remove the transplant is key because of the potential of tumors forming when stem cell-derived, insulin-producing cells – the most promising cell source for type 1 diabetes cell therapies – are used.
“When they fail or die, they need to come out,” Ma said. “You don’t want to put something in the body that you can’t take out. With our method, that’s not a problem.”
Taking inspiration from the way water beads on a spider’s web, Ma and his team first attempted to connect the islet cell-containing capsules through a string but realized that it would be better to put the hydrogel layer uniformly around a string instead.
That string: an ionized calcium-releasing, nanoporous polymer thread. The device starts with two sterile nylon sutures twisted in a helix, then folded over to facilitate the subsequent nanoporous structure coatings. Placed onto that thread is a thin layer of islet cell-containing alginate hydrogel, which adheres to the helical, nanoporous thread, similar to dew drops sticking to the spider silk. Alginate is a seaweed extract commonly used in encapsulated cell transplantation.
This thread – which the group has dubbed TRAFFIC (Thread-Reinforced Alginate Fiber For Islets enCapsulation) – was inspired by a spider’s web but, according to Ma, is even better because the hydrogel covers the thread uniformly.
“You don’t have any gaps between capsules,” he said. “With a spider’s silk, you still have gaps between the water beads. In our case, gaps would be bad in terms of scar tissue and the like.”
And since the thread is twisted and porous, the hydrogel won’t slip off as it would on a single, smooth piece of material. Fan and Silberstein were instrumental in modeling different options for the thread configuration.
This therapy would involve minimally invasive laparoscopic surgery to implant approximately six feet of hydrogel-coated thread into the patient’s peritoneal cavity.
“We only need two quarter-inch-long incisions,” Flanders said. “We inflate the abdomen with carbon dioxide, which gives us room to work, and then put in two ports – one for a scope that’s hooked to a camera, so we can see what we’re doing, and the other for a grasping device, which is how we introduce the implant.”
TRAFFIC’s large surface area promotes better mass transfer, Ma said, and diffusion is good because all the islet cells are near the surface. Current life span estimates for the thread are between six and 24 months, although more testing is necessary.
In mice, blood glucose levels were returned to normal two days after implantation of a one-inch length of TRAFFIC, and remained normal for at least three months when the experiment ended. Retrievability was tested in multiple dogs, with 10-inch samples being successfully implanted and removed laparoscopically.
Flanders, who performed surgical implantation in canines, said among the different dogs and devices tested there was either no or only minimal adhesion of the device to surrounding tissue upon removal.
This collaboration has produced a potentially game-changing medical device, he said.
“When Minglin first told me about this, I thought it was brilliant,” Flanders said. “There have been other devices sort of like this, but this one seems to have so much promise. It’s minimally reactive, it protects the islet cells, it allows them to sense glucose, they don’t attach to anything, and it can be easily removed. To me, it sounded like a win-win.”
TRAFFIC has received patent protection with the help of Cornell"s Center for Technology Licensing. Co-authors include BEE professor Dan Luo, postdoctoral researcher Wei Song, doctoral students Jason Lu and Yehudah Pardo, fiber science postdoc Dahua Shou, nutritional science professor Ling Qi and postdoc Yewei Ji.
This work was supported by the American Diabetes Association, the Cornell Technology Acceleration and Maturation Fund, and the Cornell Stem Cell Program Seed Fund. The work made use of the Cornell Center for Materials Research shared facilities, which are supported by the National Science Foundation.
Reference: http://www.pnas.org/content/early/2017/12/19/1708806115



ارسال به دوستان