Organoids help bridge gap between laboratory study and animal modeling of colorectal cancer
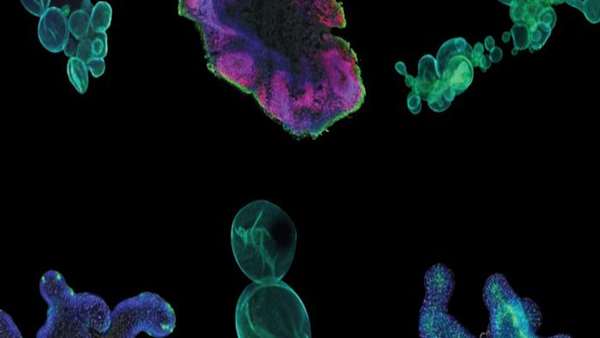
Scientists at the Wake Forest Institute for Regenerative Medicine (WFIRM) have biofabricated human colorectal cancer miniature organs, called organoids, to better understand how a tumor grows in its natural microenvironment and its response to therapies.
Scientists at the Wake Forest Institute for Regenerative Medicine (WFIRM) have biofabricated human colorectal cancer miniature organs, called organoids, to better understand how a tumor grows in its natural microenvironment and its response to therapies. This new study is the first to replicate observations of native tumor tissue in a laboratory model and validate it in the context of the whole-body physiology.
Current strategies to understand tumor progression studies are centered on the tumor cells in isolation, but do not capture the interactions between a tumor and its surrounding microenvironment. This leads to inaccuracies in predicting tumor progression and chemotherapy response.
"Tumors are products of their environment. They send signals that can have significant effects on local tissue, and they receive signals from nearby cells and tissues that can alter their progression," said Shay Soker, Ph.D., senior author of a new study published in the journal Scientific Reports.
New technologies that better show the specific properties of a tumor will have a significant effect on patient death rates and lead to development of new treatments which target the cancer, sparing healthy tissue from the side effects of chemotherapy treatments.
The WFIRM team previously developed a 3-D organoid model of the colon, complete with its unique micro-architecture, and used it to analyze colorectal cancer biopsies to identify significant changes in the miroenvironment.
The team analyzed the tumor microenvironment and corresponding "finger print" and found that samples with orderly extracellular matrix—the "glue" that holds cells together—maintained these structures. In contrast, disordered extracellular matrix allowed for a more primitive "finger print." Furthermore, these results were replicated in the context of whole-body physiology, to show for the first time that a pre-structured tumor microenvironment maintains its architecture in the laboratory. Non-traditional treatments that target the extracellular matrix might provide valuable avenues for developing new treatments or therapies that synergize with existing chemotherapeutic or radiation technologies.
By controlling cancer cell responsiveness through changes to the tumor microenvironment, lower doses of chemotherapy or radiation could become effective, thereby reducing or eliminating many of the undesirable side effects of traditional cancer therapies, as well as yielding lower tumor resistance.
"The 3-D bioengineered colon cancer constructs are a promising model for drug development and screening because they can reproduce human physiology at a high level," said Anthony Atala, MD, director of WFIRM.
Reference:https://www.nature.com/articles/s41598-020-66785-1



ارسال به دوستان