Optimization of Mechanical Tissue Dissociation Using an Integrated Microfluidic Device for Improved Generation of Single Cells Following Digestion
Dissociation of aggregated particulates is a fundamental process in diverse scientific fields, including polymer suspensions and microbeads/nanoparticles. Currently, the need for efficient disaggregation is extreme for tissue and organ samples to help facilitate powerful single-cell analysis technologies.
Traditional diagnostic methods provide information about biological traits averaged over an entire population of cells, which masks cell-to-cell variability and the presence of rare cell populations. Cell aggregate dissociation is also needed for regenerative medicine, as current methods can alter stem cell fate and viability. Therefore, continued development and refinement of rapid and efficient methods for processing tissues and cell aggregates into single cells is a significant need in the biotechnology and medical arenas. The conventional method for preparing single cells from tissue includes 1)Mincing to reduce tissue size, 2)Digesting with enzymes breaks down the extracellular matrix and/or cell-cell junctions, 3)Mechanically dissociating to release cells, 4)Filtering to remove remaining aggregates.
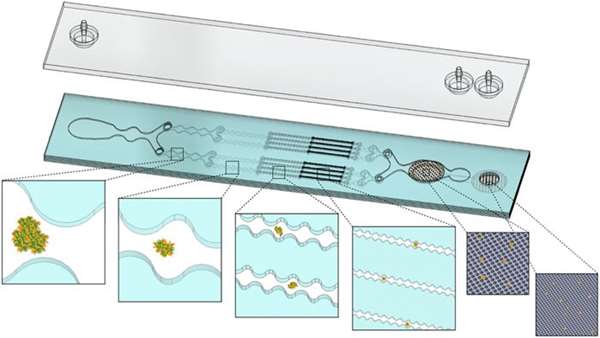
To uniformly release cells from aggregates with minimum damage, there is a need to develop and refine methods that will provide well-controlled environmental factors, including chemical exposure time and hydrodynamic shear stress level. Among these, researchers at the University of California have developed three different microfluidic devices that can perform the entire dissociation process workflow, including digestion, dissociation, and filtration. Using a strongly cohesive cell aggregate model, they found that single-cell recovery was highest using flow rates exceeding 40ml/min.
For minced and digested kidney tissue, recovery of diverse cell types was maximal using multiple passes through the channel module and only one pass through the filter module. Notably, they found that epithelial cell recovery from the optimized integrated disaggregation and filtration (IDF) device alone exceeded their previous efforts, and this result was maintained after reducing digestion time to 20 min. However, endothelial cells and leukocytes still required extended digestion time for maximal recovery. These findings highlight the significance of parameter optimization to achieve the highest cell yield and viability based on tissue sample size, extracellular matrix content, and strength of cell-cell interactions.
https://www.frontiersin.org/articles/10.3389/fbioe.2022.841046/full



ارسال به دوستان