Chimeric antigen receptor (CAR) T cell therapies approved for cancer treatment are manufactured from a patient’s own cells(autologous), entailing challenges such as high costs, inconsistent product quality, and potential treatment failure due to T cell dysfunction. In a study published in Cell Research, Hu et al. generated CAR T cells from healthy donor T cells (allogeneic), and these were successful in a clinical trial for the treatment of T cell malignancies.
CAR T cell therapies, have shown success for the treatment of various hematological malignancies. However, there are multiple challenges that need to be addressed to make this therapy widely accessible for patients. One of these challenges is the autologous (patient-derived) nature of CAR T cell products, which is associated with a complex manufacturing process, leading to disadvantages such as high costs, potential delays in treatment availability, and inconsistent product quality.
One solution to these issues could be using “off-the-shelf”(allogeneic) CAR T cells, produced with T cells derived from healthy donors. Allogeneic CAR T cells would be particularly beneficial for treating the highly aggressive T cell malignancies, because it would avoid potential product contamination with malignant T cells.
In a recent paper in Cell Research, Hu et al. generated healthy donor-derived CAR T cells targeting CD7, a transmembrane protein expressed in most T cell malignancies.
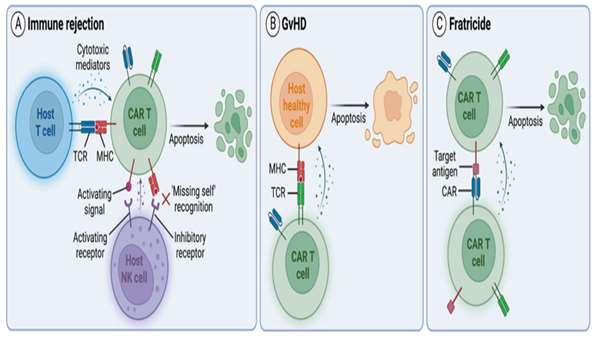
The authors introduced additional genetic modifications to overcome challenges associated with allogeneic products. First, since CD7 is expressed in healthy T cells, CD7 expression was genetically deleted to prevent CAR T cell fratricide. The T cell receptor (TCR) and its associated signaling complex CD3 were also genetically disrupted, to reduce the likelihood of GvHD caused by recognition of recipient antigens by donor TCRs. They genetically ablated human leukocyte antigen (HLA) class II expression in CAR T cells to reduce the risk of rejection by endogenous CD4+CD7‒ cells, which are resistant to CAR T cell killing due to the lack of CD7 expression.
Together, all genetic modifications resulted in potent allogeneic CD7-targeting CAR T cells (RD13-01) that were resistant to fratricide, GvHD and allo-rejection from host CD4+ T cells and NK cells.
To evaluate the clinical application of RD13-01, the authors conducted a phase I clinical trial in patients with relapsed or refractory CD7+ hematological malignancies, including T cell acute lymphoblastic leukemia, T cell lymphoma and acute myeloid leukemia.
Excitingly, RD13-01 treatment had an acceptable safety profile, with no cases of dose limiting toxicity, GvHD or immune effector cell-associated neurotoxicity syndrome. This study illustrates the potential of donor-derived CAR T cell therapies for the treatment of T cell malignancies. Further investigations are warranted to optimize antitumor efficacy and persistence to move closer towards the goal of universally accessible off-the-shelf CAR T cell therapies.
https://www.nature.com/articles/s41422-022-00745-4



ارسال به دوستان