Molecular Sleuthing Identifies and Corrects Major Flaws in Blood-Brain Barrier Model Derived from Pluripotent Stem Cells
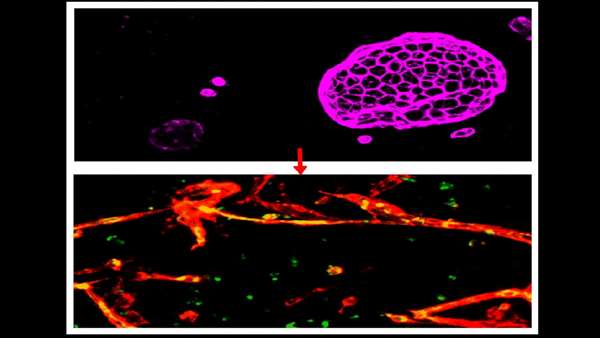
Induced brain microvascular endothelial cells (IBMECs) (top panel) cultured in 3D assume an epithelial organoid structure and express the epithelial cell marker EPCAM (purple). When they are reprogrammed by overexpression of ETV2, ERG and FLI1, they lose EPCAM expression, acquire vascular markers VE-Cad (red) and PECAM1 (green) and are able to fulfill the function of endothelial cells - forming blood vessels (bottom panel). Image courtesy of the Lis lab.
A type of cell derived from human stem cells that has been widely used for brain research and drug development may have been leading researchers astray for years, according to a study from scientists at Weill Cornell Medicine and Columbia University Irving Medical Center.
The cell, known as an induced Brain Microvascular Endothelial Cell (iBMEC), was first described by other researchers in 2012, and has been used to model the special lining of capillaries in the brain that is called the “blood-brain barrier.” Many brain diseases, including brain cancers as well as degenerative and genetic disorders, could be much more treatable if researchers could get drugs across this barrier. For that and other reasons, iBMEC-based models of the barrier have been embraced as an important standard tool in brain research.
However, in a study published Feb. 4 in the Proceedings of the National Academy of Sciences, the Weill Cornell Medicine scientists, in collaboration with scientists at Columbia University Irving Medical Center and Memorial Sloan Kettering Cancer Center, analyzed the gene expression patterns of iBMECs and found that, in fact, they are not endothelial cells—specialized cells that line blood vessels—and thus are unlikely to be useful in making accurate models of the blood-brain barrier.
“Models of key tissues and structures using stem cell technology are potentially very useful in developing better disease treatments, but as this experience indicates, we need to rigorously evaluate these models before embracing them,” said co-senior author Dr. Raphaël Lis, assistant professor of reproductive medicine in medicine and a member of the Ansary Stem Cell Institute in the Division of Regenerative Medicine at Weill Cornell Medicine. Dr. Lis is also an assistant professor of reproductive medicine in the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine at Weill Cornell Medicine.
Since 2007, researchers have known that they can use combinations of transcription factor proteins, which control gene activity, to reprogram ordinary adult cells, such as skin cells sampled from a patient, into cells resembling the stem cells of the embryonic stage of life. Researchers can then use similar reprogramming techniques to coax these cells, called induced pluripotent stem cells, to mature into different cell types—cells that can be studied in the lab for clues to normal health and disease.
The announcement in 2012 that researchers had made iBMECs, using such techniques, was exciting because the cells seemed to be one of the first highly tissue-specific cell types created with stem cell methods. The cells also seemed especially useful for research, for they were thought to be essentially the same as the vessel-lining endothelial cells that form the blood-brain barrier—which normally prevents most molecules in the blood from crossing into brain tissue. Research using iBMECs to model the blood-brain barrier, to better understand neurological diseases and develop new treatments, has been well funded and has expanded to involve many laboratories around the world.
In trying to work with iBMECs, the collaborating teams noted major unexplained discrepancies between these cells and bona fide endothelial cells, for example in their patterns of gene activity. That prompted them to investigate further, using advanced methods including the latest single-cell sequencing techniques, to rigorously compare the gene activity in iBMECs and in authentic human brain endothelial cells.
They found that iBMECs in fact have a largely non-endothelial pattern of gene activity, with little or no activity among key endothelial transcription factors or other accepted gene signatures. The cells, they found, also lack standard cell-surface proteins found in endothelial cells. Their analysis suggested that iBMECs were mistakenly classified as endothelial cells and rather represent different cell type called epithelial cells. Epithelial cells participate in the formation of a physical barrier shielding the body from pathogens and environmental insults, while supporting the transport of fluids, nutrients and waste. Present in numerous organs like intestines, lungs or skin, the epithelial barrier, unlike endothelial cells, is not equipped to transport blood.
The researchers noted that the initial studies of iBMECs almost a decade ago put more emphasis on the mechanical, barrier-like properties of these cells and less on their actual cellular identity as revealed through gene activity patterns.
Generation of various human tissues from pluripotent stem cells is one the most widely used techniques in laboratories world-wide. This study indicates that such techniques should be studied carefully to avoid misidentification of cells that could result in inaccurate outcomes.
“Previously there were fewer methods for studying gene expression profiles, and there was less understanding of the patterns that make up the identities of distinct cell types,” said co-senior author Dr. David Redmond, assistant professor of computational biology research in medicine and a member of the Ansary Stem Cell Institute in the Division of Regenerative Medicine at Weill Cornell Medicine.
The team found that by forcing the activity of three known endothelial cell transcription factors, they could reprogram iBMECs to be much more like endothelial cells.
“We don’t yet have a good ‘blood-brain barrier in a lab dish’ model, but I think we are now a step closer to that goal, and have also corrected an important misconception in the field,” said first author Tyler Lu, a research specialist in the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine at Weill Cornell Medicine.



ارسال به دوستان