Metabolic Switch Supports Human Mesenchymal Stem Cell Immunosuppression
The overarching importance of the immunomodulatory capacity of human mesenchymal stem cells (hMSCs) to their therapeutic output supposes a need to fully understand the regulatory mechanisms behind this ability in order to further develop hMSC-based therapies that can translate to the clinic.
The overarching importance of the immunomodulatory capacity of human mesenchymal stem cells (hMSCs) to their therapeutic output supposes a need to fully understand the regulatory mechanisms behind this ability in order to further develop hMSC-based therapies that can translate to the clinic. Previous studies from the laboratory of Teng Ma (Florida State University, Tallahassee, FL, USA) established that hMSC plasticity in response to a changing environment (e.g., from in vitro culture conditions to the inflamed environment in a damaged/diseased tissue in vivo) requires substantial metabolic modifications to adapt cell phenotype [1, 2] (See a relatedStem Cells Portal review article here!).
Now, the team returns with a new STEM CELLS Translational Medicine study of the potential links between hMSC immunosuppression and metabolic activity. Interestingly, Liu et al. establish that immune “licensing” of hMSCs by exposure to the pro-inflammatory cytokine interferon‐gamma (IFN‐γ) [3] promotes the modification of cell metabolism towards glycolysis to support the secretion of immunosuppressive factors [4].
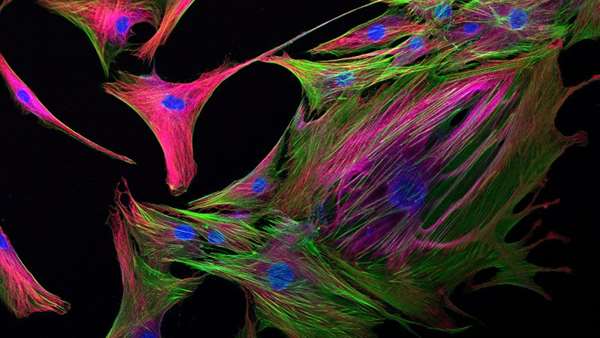
Initial analysis revealed that naïve bone marrow-derived hMSCs employ mitochondria-based oxidative phosphorylation for energy production and express low levels of the kynurenine and Prostaglandin E2 (PGE2) immunosuppressive factors. Treatment of hMSCs with IFN‐γ as a means to “kick-start” their immunosuppressive activities triggered the activation of the Akt/mammalian target of rapamycin (mTOR) signaling pathway activation, which functions in nutrient sensing and maintaining cellular and metabolic homeostasis in response to external stimuli [5]. Subsequently, immune licensed hMSCs altered their metabolism, shifting from mitochondrial respiration to cytoplasm-based aerobic glycolysis, which prompted an overall increase in the metabolic potential of hMSCs. Importantly, this metabolic switch supported the transformation of immune licensed hMSCs into an immunosuppressive phenotype and led to the enhanced secretion of immunosuppressive factors (kynurenine and PGE2), with additional reductions in oxidative phosphorylation levels further enhancing their production, and the suppression of immune T cell proliferation.
As expected from the IFN‐γ-mediated immune licensing-mediated metabolic switch in hMSCs, the authors observed increased glucose consumption and elevated expression of key glycolysis enzymes, as well as reduced mitochondrial electron transport activity and increased production of mitochondrial reactive oxidative species (mROS). Interestingly, the authors linked elevated mROS levels to the increase in glycolysis and immunosuppressive factor secretion, suggesting that both mROS and Akt/mTOR pathways control the metabolic switch observed in response to immune licensing.
Overall, this study suggests that the metabolic state of hMSCs influences their immunomodulatory properties, a finding that agrees with those found during the activation of anti‐inflammatory immune cells [6]. The authors note that altering metabolic status in hMSCs offers an immediately translational means of controlling immunomodulatory properties and improving their therapeutic efficacy. The authors of this fascinating study hope to now delineate other regulatory mechanisms that operate in IFN‐γ licensing in order to advance immunomodulation by hMSCs towards clinical application.



ارسال به دوستان