Genetic Modification of Cytokine Signaling to Enhance Efficacy of CAR T Cell Therapy in Solid Tumors
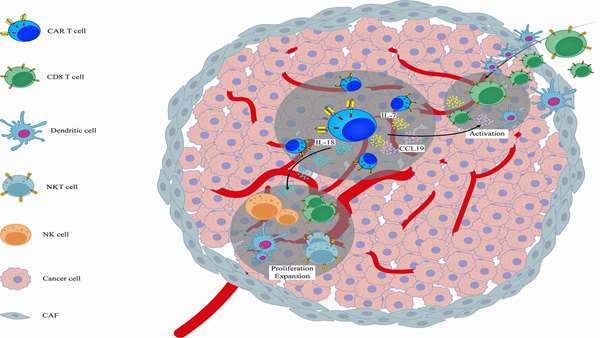
Chimeric antigen receptor (CAR) T cell therapy has shown unprecedented success in treating advanced hematological malignancies. Its effectiveness in solid tumors has been limited due to heterogeneous antigen expression, a suppressive tumor microenvironment, suboptimal trafficking to the tumor site and poor CAR T cell persistence. Several approaches have been developed to overcome these obstacles through various strategies including the genetic engineering of CAR T cells to blunt the signaling of immune inhibitory receptors as well as to modulate signaling of cytokine/chemokine molecules and their receptors. In this review we offer our perspective on how genetically modifying cytokine/chemokine molecules and their receptors can improve CAR T cell qualities such as functionality, persistence (e.g. resistance to pro-apoptotic signals) and infiltration into tumor sites. Understanding how such modifications can overcome barriers to CAR T cell effectiveness will undoubtedly enhance the potential of CAR T cells against solid tumors.
Genetically modifying cytokine/chemokine signaling pathways is an appealing approach to enhance the therapeutic properties of CAR T cell therapy for solid tumors. It is anticipated that new generations of cytokine/chemokine-gene-modified CAR T cells could effectively target/engage endogenous immune system to synergistically improve overall antitumor immunity and additionally prevent the appearance of antigen-negative tumor variants, and thereby, tumor relapse in the context of CAR T cell therapy. However, CRS and ICANS incidences remain main safety concerns as CRS and ICANS are potentially fatal adverse effect of CAR T cell therapy and genetic modifications of cytokines/chemokines that enhance CAR T cell function could exacerbate CRS and ICANS. To date, nearly all of studies have only investigated constitutive expression of single chemokine/cytokine. Thus, generation of CAR T cells engineered with an inducible chemokine/cytokine platform not only can increase efficacy but also guarantee safety of CAR T cell therapy.
Due to the fact that cytokine/chemokine gene expression profile can be various between different individuals or even at different stages of same tumor in an individual, hence; analysis of cytokine/chemokine gene expression signature in TME before CAR T cell therapy could aid scientists to individualize cytokine engineered-based CAR T cell therapy. Although genetic modification of cytokine signaling in CAR T cells have shown promising clinical results in both hematologic and non-hematologic cancers (Table 2), it seems combination of different genetic modification approaches could be beneficial as some of cytokines are immuno-inhibitory and others are immuno-stimulatory. Thus, modification of immunoinhibitory pathways using gene editing technologies (e.g. CRISPR/Cas9) or use of truncated cytokine receptors or cytokine traps alongside with overexpression of immune-stimulatory cytokines may reprogram TME and significantly improve the efficacy of CAR T cell therapy.
Finally, it seems that a more comprehensive understanding of the relevant cellular and molecular adaptations to tumor cells and immunological processes in their surrounding microenvironment will help us develop new generations of cytokine/chemokine-gene-modified CAR T cells that are more potent in overcoming the challenges of solid tumors.
Ref:



ارسال به دوستان