Direct conversion of human umbilical cord mesenchymal stem cells into retinal pigment epithelial cells for treatment of retinal degeneration
Age-related macular degeneration (AMD) is a leading cause of visual impairment worldwide. It is characterized by visual function damage caused by apoptosis and loss of function of retinal pigment epithelial cells (RPE) and retinal photoreceptor cells. AMD often leads to severe visual impairment and eventual blindness, which seriously affect the quality of life of patients. In recent years, stem cell-based treatments have been applied in the clinic and show obvious therapeutic outcomes.
researchers have demonstrated that MSC subretinal space transplantation can significantly inhibit photoreceptor cell apoptosis and delay the loss of vision in animal AMD model. However, the differentiation ability of MSCs transplanted into the subretinal space in rat AMD models is still controversial, and transplantation site also affects the outcomes of MSC-based therapy for retinal degeneration. Therefore, the differentiation of MSCs into RPE cells in vitro and subsequent transplantation into the subretinal space is expected to improve the outcome of cell therapy.
Small molecules and/or RPE-conditioned medium were used to induce the differentiation of MSCs into RPE cells in vitro. Whereas, the environment in retina of AMD patients is hostile. Clinical evidence demonstrates that RPE cells undergo epithelial-to mesenchymal transition (EMT) in AMD, and the level of transforming growth factor-β (TGF-β), an inducer of EMT, is elevated in the retina of patients with AMD compared with that in normal control eyes, suggesting that transplanted RPE cells derived from MSCs are very likely to undergo EMT, subsequently reducing the therapeutic effect of transplanted cells. Therefore, if RPE cells derived from MSCs exhibit anti-EMT ability, the efficacy of MSC-based therapies will be greatly improved.
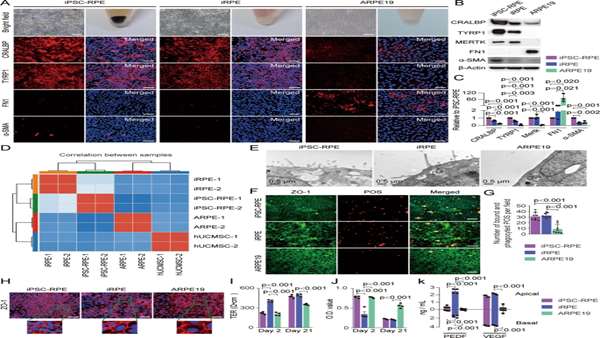
It has been reported that key transcription factors can transdifferentiate one kind of cells into another lineage of cells.Based on this evidence,researchers screened a combination of key transcription factors that were able to transdifferentiate hUCMSCs into RPE-like cells, termed induced RPE (iRPE) cells. hUCMSCs were transdifferentiated into iRPE cells by five key TFs: CRX, NR2E1, C-MYC, LHX2, and SIX6.
The iRPE cells had characteristics comparable to those of induced pluripotent stem cell-derived RPE (iPSC-RPE) cells and had anti-EMT properties.The gene expression patterns and functions of iRPE cells are similar to those of iPSC-RPE cells, and iRPE cells have significantly better therapeutic function than hUCMSCs.
CRX is thought to be the key TF for photoreceptor cell fate determination, and C-MYC plays an important role in the control of cell proliferation, growth, differentiation, apoptosis, survival, and stem cell self-renewal. LHX2 regulates the expression of visual cycle genes in RPE cells. LHX2 and C-MYC are essential for maintaining mature RPE function.
Researchers confirmed that transplantation of iRPE cells derived from hUCMSCs markedly improved therapeutic outcomes in an animal model of AMD, which will enhance the clinical application value of hUCMSCs.
A limitation of this study is that Researchers used retroviruses to randomly integrate the five TFs into the chromosomes of hUCMSCs for reprogramming the cells into iRPE cells. Although we did not observe tumorigenesis after transplantation of iRPE cells in nude mice, there are still risks with regards to future clinical use. Furthermore, because lots of the grafted cells died 6 weeks post-transplantation, an appropriate immunosuppression scheme is required to prolong the survival of grafted cells in animal models.



ارسال به دوستان