CRISPR Nanoparticles Repair Duchenne Muscular Dystrophy Gene
Scientists have developed a gold nanoparticle technology for delivering the CRISPR/Cas9 gene-editing system to cells that, when tested in the mdx mouse model of Duchenne muscular dystrophy (DMD), repaired the faulty DMD gene, leading to improved strength and agility and reduced fibrosis
Scientists have developed a gold nanoparticle technology for delivering the CRISPR/Cas9 gene-editing system to cells that, when tested in the mdx mouse model of Duchenne muscular dystrophy (DMD), repaired the faulty DMD gene, leading to improved strength and agility and reduced fibrosis. Professor Niren Murthy, Ph.D., the University of California, Berkeley (UC Berkeley) researcher who led development of the CRISPR-Gold platform, suggested to GEN that human clinical gene-editing trials using the system could feasibly start within the next few years.
CRISPR-Gold uses gold nanoparticles to encapsulate all of the elements needed for CRISPR/Cas9 gene editing and deliver them directly to cells. Initial tests in mice achieved levels of gene correction that could demonstrate significant clinical benefit if they can be reproduced in humans, professor Murthy told GEN. “We think a clinical trial for therapeutic gene editing with CRISPR-Gold and its derivatives could happen within 5 years.” However, he added, its not clear whether initial trials will be in DMD, or another disease. “DMD is a very compelling first clinical application of the CRISPR-Gold technology, however there are several others, and it is still too soon to say what the first clinical application will be.”
The CRISPR-Gold platform has been developed by professor Murthy’s team in collaboration with fellow UC Berkeley bioengineering professor Irina Conboy, Ph.D., and colleagues in the U.S. and Japan. The researchers report on the development of and initial in vitro and in vivo studies with the platform in Nature Biomedical Engineering, in a paper entitled, “Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA In Vivo Induces Homology-Directed DNA Repair.”
There are two basic categories of gene editing to which CRISPR/Cas9 could potentially be applied in a therapeutic setting, the authors explain. The first, based on nonhomologous end joining, aims to permanently silence disease-causing genes by inducing indel mutations. The second, known as homology-directed repair (HDR), aims to correct disease-causing gene mutations back to their wild-type sequences.
Although the use of CRISPR/Cas9 to correct mutations through HDR has the potential to “revolutionize” the treatment of genetic diseases, in practice, the approach is challenging because HDR-based therapeutics would need to simultaneously deliver the Cas9 protein, the guide RNA (gRNA) that directs Cas9 to the mutated gene, and donor RNA, the researchers note. The most advanced of the current potential delivery systems based on adeno-associated viruses (AAVs) can’t be used universally because a large proportion of the human population has preexisting immunity toward AAVs, while AAV-based Cas9 delivery also has the potential to cause significant off-target genomic damage. There are also challenges associated with the direct delivery of Cas9 ribonucleoprotein (Cas9-RNP) using vehicles such as lipofectamine and polyethylenimine.
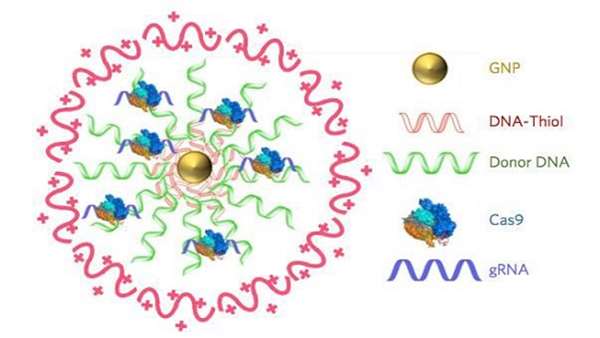
“We needed to develop a delivery vehicle that could simultaneously deliver Cas9 protein, gRNA, and donor DNA,” explained professor Murthy, whose lab is focused on developing new drug-delivery vehicles. "We felt that this could be done if we had a nanoparticle that could encapsulate all three of these components,” he tells GEN. The resulting CRISPR-Gold delivery system comprises gold nanoparticles (GNPs) conjugated with DNA, which are complexed with the donor DNA, Cas9-RNP, and an endosomal disruptive polymer, poly[N-(N-(2-aminoethyl)-2-aminoethyl] aspartamide [PAsp(DET)]. This is an “ingenious lysosome-disruptive polymer coat to ensure delivery of the cargo to the cytoplasm and then nuclei,” says Dr. Conboy to GEN. When injected locally, the nanoparticles are recognized by cells and are readily internalized via endocytosis. The PAsp(DET) polymer then triggers endosomal disruption, so that the CRISPR-Gold nanoparticles are discharged into the cytoplasm, where the Cas9-RNP and donor DNA are released from the nanoparticle gold core.
“We used gold nanoparticles, because gold can efficiently react with thiol-modified DNA, and this allowed us to make gold-DNA conjugates, which bind Cas9/gRNA complexes,” professor Murthy continued. “CRISPR-Golds main benefit is that it can encapsulate of all the components needed for gene repair and that the Cas9 protein encapsulated is still active.”
In the Nature Biomedical Engineering paper the UC Berkeley-led team describes use of the CRISPR-Gold system to deliver all the components required for CRISPR/Cas9-mediated HDR directly to a wide variety of therapeutically relevant cell types, including human embryonic stem cells, human induced pluripotent stem cells, and bone-marrow derived dendritic cells, as well as myoblasts from the mdx mouse model. Using the CRISPR-Gold platform, HDR efficiency was typically 3% to 4%, and there was minimal off-target DNA damage.
Having demonstrated the technology in cells, the team then turned to the mdx mouse model of DMD as a therapeutic test case for CRISPR-Gold. In humans, the muscle-wasting disease is caused by mutations in the dystrophin gene, and about 30% of patients have single base mutations or small deletions that could feasibly be corrected using HDR-based strategies. However, the only current gene-editing strategy for treating the disease is based on nonhomologous end-joining–induced exon skipping, which generates a shortened form of dystrophin that is only partially effective. Exon-skipping essentially just changes one mutation in the gene to another less deleterious mutation, professor Conboy notes.
In contrast, a single intramuscular injection of CRISPR-Gold in young mdx mice, simultaneously with cardiotoxin (CTX) administration to activate muscle stem cell proliferation, led to 5.4% of the mutated dystrophin gene being corrected back to wild type. “CRISPR-Gold is the first example of a nonviral delivery vehicle that could deliver the Cas9 protein, gRNA and donor DNA in vivo and correct genes via homology-directed repair,” professor Murthy claims. “A previously reported, viral-based non-HDR exon-skipping approach only achieved a gene-editing rate of 2%. However, it is very hard to directly compare because we did HDR and they used a gene-deletion approach,” he comments to GEN. “We also had less side effects (non-specific DNA damage and mutations elsewhere in the genome), as compared to the previous technologies,” professor Conboy pointed out.
Further analysis of the muscle tissues injected with CRISPR-Gold demonstrated robust dystrophin protein expression. And when compared with control animals, the CRISPR-Gold-treated mice showed a two-fold improvement in hanging time in a four-limb hanging test. Encouragingly, when the team carried out a deep sequencing analysis, they found that the degree of off-target DNA damage caused by CRISPR-Gold was no greater than the level of sequencing error (0.005–0.02%) found in a typical cell that hadn’t been exposed to CRISPR-Gold. “These results demonstrate that CRISPR-Gold can induce HDR in muscle tissue with minimal off-target genomic damage, effectively edit the dystrophin mutation in mdx mice to the wild-type sequence, and improve animals strength under clinically relevant conditions,” the researchers write.
They also tested for potential immunogenicity by measuring blood cytokine levels in treated animals, 24 hours and 2 weeks after CRISPR-Gold injection. The results showed no sign of inflammatory cytokine upregulation, and there was not weight loss, even after multiple injections, “suggesting that CRISPR–Gold can be used multiple times safely and that it has a high therapeutic window for gene editing in muscle tissue." In fact, compared with virus-based CRISPR-Cas9 delivery, “nanoparticles are less immunogenic, less toxic and much cheaper to produce,” Dr. Murthy stated.
Achieving significant clinical benefits in human DMD patients would require about a 5-20% gene correction rate, he indicated to GEN. “We are basically at that efficiency in mice after a single injection. However mice and humans are very different, and we don’t know what the gene-editing efficiency in humans will be. We want to increase gene-editing efficiency of the particles before we translate them.”
One of the major benefits of gene editing is that therapy would be permanent. “We anticipate that a patient could be treated for life, after a few injections with CRISPR-Gold,” professor Murthy suggested. “Injections would have to done in numerous places in the muscle, however, because it is permanent, the injections would not need to be done on a chronic basis.”
The ability of CRISPR-Gold to deliver all the CRISPR-Cas9 elements to a variety of cell types means that the technology also has potential widespread therapeutic applications. “We are therefore collaborating with numerous laboratories and investigating its therapeutic potential for several diseases,” he added. “Any tissue disease that can be accessed with a direct injection is a potential candidate for a CRISPR-Gold therapy.”
Professor Murthy, together with UC Berkeley co-authors Kunwoo Lee, Ph.D., and Hyo Min Park, Ph.D., have founded a company, GenEdit, to develop the CRISPR-Gold technology for human therapeutic applications. “GenEdit was formed about 16 months ago,” says Murthy. “GenEdit is heavily focused on new delivery vehicles for the Cas9 protein, and is developing platform technologies for this.The company has raised seed funding from well established venture capital companies. Partnerships with other companies are being considered.”
In parallel with the establishment of GenEdit, ongoing work at the UC Berkeley laboratories of Drs. Murthy and Conboy is focused on developing the next generation of particles that could deliver CRISPR-Gold from the bloodstream into tissues, and preferentially target adult stem cells. “We are heavily focused on improving the gene-editing efficiency of the particles and for making them effective after an intravenous injection,” Dr. Murthy commented.
“We are working on the NextGen particles that could be injected intravenously and will deliver CRISPR to tissues in need,” professor Conboy added. “ … we aim to target the tissue stem cells, because CRISPR is effective in dividing cells and stem cells not only produce new healthy tissue, but self-renew to make more of the wild-type gene edited stem cells for future tissue repair.”
Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nature Biomedical Engineering (2017). doi:10.1038/s41551-017-0137-2



ارسال به دوستان