CRISPR Gene Therapy via Skin Grafts Treats Obesity and Diabetes in Mice
Genetically engineered skin cells grafted onto mice can treat the animals’ diabetes and obesity, according to new research published August 2, 2017 in Cell Stem Cell.
Genetically engineered skin cells grafted onto mice can treat the animals’ diabetes and obesity, according to new research published August 2, 2017 in Cell Stem Cell.
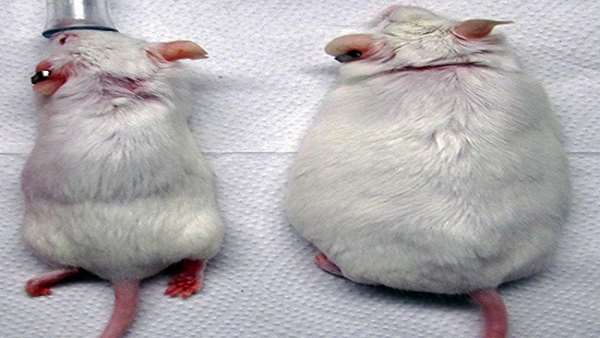
Researchers edited skin stem cells from newborn mice using CRISPR-based technology so that the cells secreted a peptide that regulates blood sugar. Transplanting the cells onto mice showed the grafts increased insulin secretion and reversed weight gain from a high-fat diet, as well as overturned insulin resistance. The result is a small step toward developing a safe and durable gene therapy to treat diabetes in humans.
“We’ve had this idea for a long time, so it’s exciting to see that, indeed, it can work to deliver therapeutics,” coauthor Xiaoyang Wu, a stem cell biologist at the University of Chicago, tells GEN.
In the study, Wu and colleagues worked with skin because it is a large organ and easily accessible. The cells multiply quickly and are easily transplanted. And, transplanted cells can be removed, if needed. “Skin is such a beautiful system,” Wu says, noting that its features make it a perfect medium for testing gene therapies.
The team worked with the gene that produces glucagon-like peptide 1 (GLP-1), a hormone that stimulates the pancreas to secrete insulin. The additional insulin takes excessive glucose out of the bloodstream, which regulates complications from diabetes. The hormone can also decrease appetite. Using the genetic engineering tool CRISPR, the team inserted a mutation, adding an antibody fragment to the gene that would make the GLP-1 last longer in the blood and an additional modification to the targeting vector that would also attach an inducible promoter. This switch turns the gene on, as needed, to make more GLP-1. The switch would be triggered by the administration of the antibiotic doxycycline.
Wu and colleagues then inserted the altered gene into skin cells and grew the cells in a culture. Once the skin cells had grown into multiple layers, the team transplanted the patches onto mice with intact immune systems. Surprisingly, the mice didn’t reject the grafts—a feat in itself—since human skin transplants are far more advanced than mice grafts, partly due to the animal’s furry skin.
Next, the team fed the mice small amounts of doxycycline. As a result, the animals released GLP-1 into the blood and had higher levels of insulin and lower levels of glucose. When fed a high-fat diet, the mice gained weight and became obese. But when the mice also were fed doxycycline so they secreted GLP-1, they gained less weight, showing the gene therapy was successful.
“This kind of therapy could be potentially effective for many metabolic disorders,” Wu says. The grafts could be used in patients who can’t process protein or in individuals with hemophilia. The team is now testing the gene-therapy technique in combination with other medications.
Reference: http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30274-6



ارسال به دوستان