Achieving Efficient Manufacturing and Quality Assurance through Synthetic Cell Therapy Design
New methods to manipulate gene and cell state can be used to engineer cell functionality, simplify quality assessment, and enhance manufacturability. These strategies could help overcome unresolved cell therapy manufacturing challenges and complement frameworks to design quality into these complex cellular systems, ultimately increasing patient access to living therapeutics.
New methods to manipulate gene and cell state can be used to engineer cell functionality, simplify quality assessment, and enhance manufacturability. These strategies could help overcome unresolved cell therapy manufacturing challenges and complement frameworks to design quality into these complex cellular systems, ultimately increasing patient access to living therapeutics.
Main Text
Design for Quality, Engineer for Manufacturability
Cell therapies, in which patients are treated with living cells, are being developed to cure a wide range of devastating diseases. Treating patients with living cells whose functionality is determined by cellular properties sensitive to small environmental changes poses significant challenges for manufacturing these complex cell therapy products (CTPs). We recently discussed how the quality by design (QbD) framework could be applied to mitigate risk in the development of manufacturing solutions for CTPs (Lipsitz et al., 2016). This framework can guide CTP manufacturing process development by increasing our understanding of the attributes critical for assuring product quality and safety and by identifying and controlling the sources of variability in those attributes. However, CTPs pose product and process development barriers that remain challenging, even in the face of rigorous implementation frameworks. These include ensuring robust product functionality, quantitative and rapid cell characterization, and cost-effective manufacturability.
Typically, the main criteria for identifying and developing prospective CTPs has been phenotypic alignment with clinically relevant in vivo counterparts combined with simple and reliable access to the desired cells or some ability to grow or process the cells ex vivo. This bottom-up approach does not typically enable intentional targeting of specific unmet clinical needs, but rather encourages testing of readily available cells in a variety of potential clinical applications. Our field has spent too much time and effort looking for clinical applications for cells we happen to be able to grow. A top-down approach to defining desired product profiles (functionality, identity and purity, and manufacturability) required to treat a specific indication, and to implementing technologies to generate these a priori designed CTPs, should accelerate CTP impact and efficacy (Figure 1). The target product profile, a tool endorsed by the pharmaceutical industry and the US FDA for 10 years (see Web Resources), continues to be a strategic development tool for structuring process development. Although originally intended for pharmaceutical development, this tool should also serve as the basis for the design of high-quality, manufacturable synthetic CTPs.
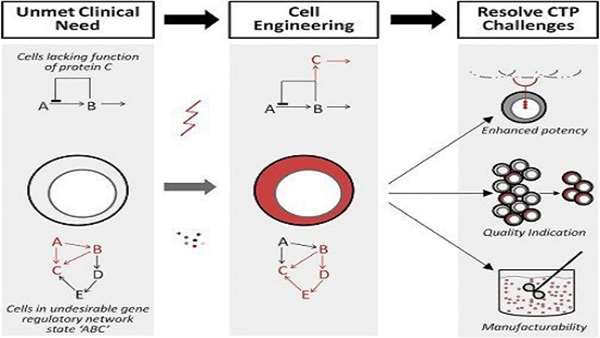
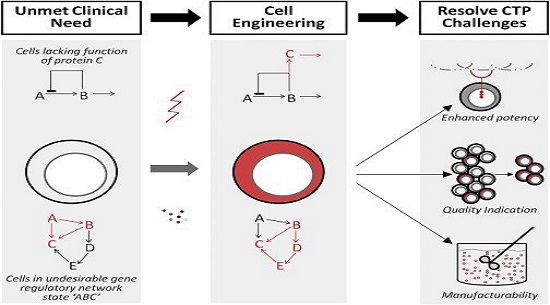
Figure 1
Therapeutics Designed from Readily Available Cell Types Can Fail to Address Unmet Clinical Need
Therapeutics may lack a needed function (the function of protein “C”) or exist in an undesirable gene regulatory network state (state “ABC”). Tools to engineering cells include gene manipulation (e.g., inserting gene “C” into the cell) or cell state manipulation (cytokine-induced shift to gene regulatory network “BCDE”). These strategies can resolve CTP challenges including designing new CTP functionality (such as a new receptor on an immune cell for a cancer-specific antigen), simplifying quality assessment by indicating or selecting for a desired cell population in a heterogeneous culture, and enhancing cell manufacturability by generating universal-donor or robustly expanding cell populations. These modifications are illustrated in red.
Protein manufacturers have engineered producer cell lines and proteins for enhanced protein yields by including switching mechanisms controlling key genes, adapting cells to minimal growth factor requirements, and engineering proteins for simplified downstream processing (Figure 2). In cell therapy, few of these solutions have been conceptually adapted, likely due to a lack of understanding of the fundamental biology governing behavior of therapeutic cells together with concern about the creation of “artificial” cells that do not fit current regulatory frameworks. In biopharma, cell engineering is at least one step removed from the product, as the engineered cell produces the therapeutic protein. However, in cell therapy, the engineered cell itself will directly interact with a patient, justifying additional safety considerations. Recent developments and clinical successes in cell engineering motivate re-examining this paradigm to accelerate CTP development. Herein we discuss progress, opportunities, and regulatory perspectives related to engineering new cell types with user-defined properties for enhanced therapeutic functionality, simplified quality assessment, and enhanced manufacturability.
Figure 2
Complexity and Extent of Synthetic Properties of CTPs Compared to Other Therapeutics
Synthetic cell therapy via cell engineering represents a logical next step to which other therapeutic types have progressed. Just as small-molecule drugs progressed from naturally occurring products through synthesized drugs to synthetic, biomaterial integrated drugs, so too have cell therapies progressed from minimally manipulated cells to cultured cells, and now to engineered cells. A key difference between these strategies is the increased complexity of engineered cells relative to small-molecule drugs and most biologics. However, ample precedent for the progression to synthetic therapeutics has been set in other advanced therapies including a variety of antibody-drug conjugates and engineered protein products.
Achieving Efficient Manufacturing and Quality Assurance through Synthetic Cell Therapy Design. Cell Stem Cell. Volume 20, Issue 1, p13–17, 5 January 2017. DOI: http://dx.doi.org/10.1016/j.stem.2016.12.003



ارسال به دوستان