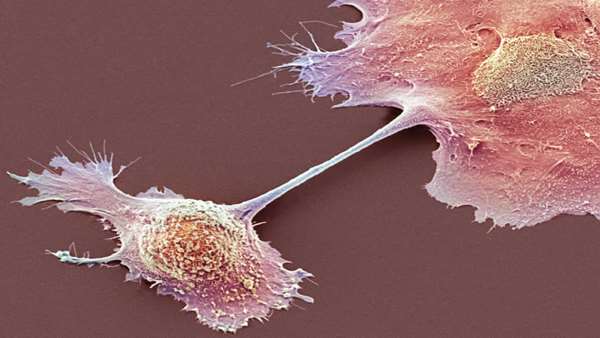
Researchers have managed to tame pancreatic cancer in a woman whose cancer was far advanced and after other forms of treatment had failed.
The experiment that helped her is complex and highly personalized and is not immediately applicable to most cancer patients. Another pancreatic cancer patient, who received the same treatment, did not respond and died of her disease.
Nonetheless, a leading journal — The New England Journal of Medicine — published a report of the study on Wednesday.
Dr. Eric Rubin, the journal’s editor in chief called the proof of concept experiment “an important step along the way” to devising similar treatments that might be applicable to lung, colon and other cancers.
The experiment involved genetically reprogramming the patient’s T cells, a type of white blood cell of the immune system, so they can recognize and kill cancer cells. The technique was developed by Eric Tran and Dr.
RomLeidner of the Earle A. Chiles Research Institute, a division of Providence Cancer Institute in Portland, Ore.
To turn a cancer patient’s T cells into a living drug, the researchers had to overcame serious challenges. Pancreatic cancer is one of the most difficult to treat. While new treatments have allowed patients with other cancers to live longer and to have a better quality of life, pancreatic cancer has stubbornly resisted these advances. Less than 10 percent of patients live past five years.
For most patients, said Dr. William Jarnagin, a pancreatic cancer specialist at Memorial Sloan Kettering Cancer Center, who was not involved in the current experiment, the cancer has already spread by the time it is discovered. Even when the tumors are caught in the pancreas and surgically removed, about 85 percent of patients have recurrences.
The technique described in the new paper, “is not off-the-shelf,” Dr. Tran said. He added that “it takes specialized facilities and expertise to manufacture the T cells.”
But, Dr. Leidner said, “the beauty of it” is that thereprogrammed T cells will only attack cancer cells. Other cells will be left alone.
The first problem in trying to entice T cells to kill cancer cells is that mutated proteins that drive the growth of cancer are hidden inside cells.
There is, though, a hint to the immune system that the cancer cells are abnormal. They contain fragments of mutated cancer proteins on their surface, “kind of like molecular bread crumbs,” Dr. Leidner said. The challenge was to get T cells to see those crumbs.
The solution employed was to collect the patient’s own T cells and genetically modify them in the lab to recognize and attach to those bits of mutated proteins. Then the T cells were infused back into the patient.
In this case the target was KRAS, a mutated protein implicated in 25 percent of all cancers, including about 95 percent of pancreas cancers, 40 percent of colon cancers and a third of lung cancers.
“Folks have been trying to target KRAS immunologically for more than 20 years,” said Dr. Robert Vonderheide, a pancreatic cancer specialist and director of the University of Pennsylvania’s Abramson Cancer Center.
The mutated KRAS gene “is such a bull's-eye,” Dr. Vonderheide said, that killing cancer cells by attacking cells with KRAS mutations has “major implications.”
But the encouraging result comes with some real caveats. For starters, it is not clear why the other patient who died did not respond to the therapy.
Dr. Elizabeth Jaffee, a pancreatic cancer specialist at Johns Hopkins Medicine also highlighted the location of the patient’s metastases, or where the cancer had spread to. Metastases arose only in the patient’s lungs. Most pancreatic cancer patients have metastases in their liver that are more difficult to treat.
“I would like to see liver lesions go away,” Dr. Jaffee said.
Kathy Wilkes, the patient who was successfully treated, is 71 and lives in Ormond-by-the-Sea, Fla. It is too soon to know if the cancer will come roaring back.
Ms. Wilkes’ cancer was severe.
“This lady had had all of the available treatments and was failing,” said Dr. Jarnagin, who did not treat Ms. Wilkes but reviewed her case. Usually, in such cases, the cancer has developed resistance to any additional treatments.
“For most in that situation the cancer is going to win — soon,” he said.
Ms. Wilkes first noticed symptoms that were later attributed to pancreatic cancer in 2015. She was tired, lethargic and had bouts of intense pain. At first, tumors did not appear on scans. But by early 2018, a tumor showed up — a 3.5-centimeter mass in the head of her pancreas.
She had chemotherapy followed by a grueling operation — the Whipple procedure — in which surgeons remove the head of the pancreas, the first part of the small intestine, the gallbladder and the bile duct. Then she had more chemotherapy, followed by radiation and even more chemotherapy.
The cancer was gone from her pancreas, but nodules appeared in her lungs — metastases. The chemotherapy and radiation continued throughout 2018.
“I just went through with it. I certainly wasn’t ready to die,” Ms. Wilkes said. “I had this voice inside saying, ‘You can best this one.’”
She entered an immunotherapy clinical trial in Pittsburgh in 2020. Her tumors shrank at first but then grew back.
She had the genes in her lung metastases sequenced, and when she learned that they were being driven by a particular KRAS mutation, she started searching for clinical trials.
She found Dr. Tran, a leader in using T cells to attack cancer mutations, and called him. Traveling to Oregon for treatment was no problem, she said. She used to live in Oregon and had family there.
On June 14, 2021, her treatment began. A month later her lung tumors had shrunk by 67 percent and were too small to biopsy. By September they appeared to have shrunk more. She had another scan last week, on May 25. The spots on her lungs had not changed. Perhaps they now consisted of dead cells.
https://www.nytimes.com/2022/06/01/health/pancreatic-cancer-treatment.html



ارسال به دوستان