At a far distant point in Earth’s ancient past, two separate, single-celled life forms — an archaeon and a bacteria — became one in an act either of symbiosis or enslavement, depending on which microbiologist you ask.
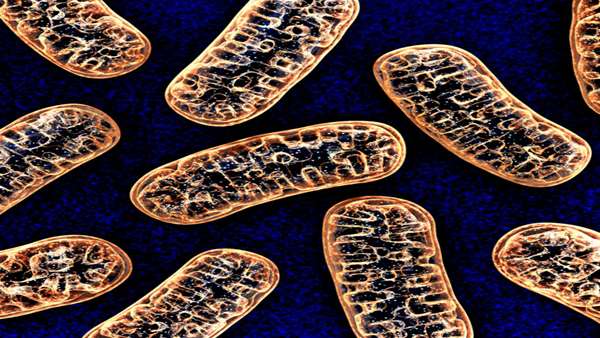
And over the next 2 billion or so years, that bacteria evolved to be the mitochondria that power nearly every cell in the human body. These capsule-shaped organelles don’t just turn oxygen and nutrients into chemical energy. They also metabolize cholesterol and synthesize hormones and neurotransmitters. They’re the reason we can walk up stairs, run long distances, jump and swim and laugh and love. Without them, we’d still be sliming around a primordial mudpot somewhere.
Which is why, when things go wrong with one’s mitochondria, the consequences are often calamitous. One way that can happen is for a large section of mitochondrial DNA to spontaneously disappear between one generation and another. It’s like losing a chapter in the wiring manual for the cell’s power plant. The resulting mitochondrial deletion disorders, known as SLSMDs, usually manifest during the second decade of a child’s life as progressive, multisystem failures including hearing and vision loss, muscle weakness, gastrointestinal and cardiac issues, dementia, and early death.
“These children are extremely weak compared to their peers,” said Elad Jacoby, a pediatric hematologist at Sheba Medical Center in Tel Aviv who led the study. One 6-year-old boy had to be pushed around the hospital in a stroller, another girl was bedridden and unable to even sit up. A few weeks following the treatment, she gained the ability to stand. The boy began to run around and stay awake long enough to eat more and put on weight.
“These are very soft measures that are hard to report, but we did see major improvements to the quality of life in almost all of these children,” Jacoby said.
If the results hold up in a clinical trial expected to start next year, mitochondrial augmentation therapy, also known as mitochondrial transplantation, could provide a way to treat not just rare genetic diseases, but a long list of other conditions and injuries arising from mitochondrial damage.
“This is a good first study to show that mitochondrial transplantation is safe in patients with these gene deletion disorders,” said James McCully, a cardiac surgeon at Boston Children’s Hospital and researcher at Harvard Medical School who was not involved in the study. “It’s nice to see someone else doing this work; it shows the whole field is moving forward.”
In 2009, a team led by McCully provided the first evidence, in rabbits, that mitochondrial transplantation could help damaged hearts recover from injury. For decades, he’d watched what happened to patients when the blood supply
to their hearts got cut off. No matter what he and other surgeons tried to do to protect them, the mitochondria in those oxygen-starved tissues got damaged beyond repair. As a result, the hearts never fully recovered, even after the blood supply came back.
One day, he wondered what would happen if you injected some healthy mitochondria from elsewhere in the body into a damaged heart. They tried it with a pig, and a few minutes later its heart had regained its strength — beating more rapidly and pushing more blood through the animal’s body. “It was quite dramatic,” McCully said. “But if it weren’t for that visible response, I’m not sure it would have caught on.”
When McCully’s team published the results, many scientists didn’t believe that mitochondria could make their way into new cells and jump into their energy assembly lines.
“It was a very controversial idea 10 years ago,” said Natalie Yivgi-Ohana. That’s when the Israeli-born biochemist began looking for investors to support a new company focused on developing mitochondria-based medicines. She had trained as an embryologist and seen how combining mitochondria from one egg with the genetic material of another — a technique sometimes called three-parent IVF — had opened the door to preventing mitochondrial diseases. In 2012, she cofounded Minovia to push forward the idea that you could also use mitochondria to treat such disorders.
Initially, the Haifa-based biotech planned to prove the
concept by injecting mitochondria directly into the eyes of people with an inherited eye disease known as Leber hereditary optic neuropathy. But in 2016, she was approached by the family of a young boy with Pearson syndrome — an extremely rare mitochondrial deletion syndrome that affects less than 100 people worldwide. It comes on early, usually in the first year of life, and leads to severe bone marrow failure, requiring patients to receive frequent blood transfusions. Because it’s caused by a deletion that arises spontaneously, unlike many other mitochondrial diseases, the mothers of such patients don’t carry the mutation. That created an opportunity to use them as mitochondrial donors.
Minovia approached the hospital where the patient was being treated, Sheba Medical Center, which specializes in Pearson and other mitochondrial diseases, about running a trial. It began enrolling in mid-2019. But in the meantime, the company worked with Jacoby and other members of Sheba’s medical teams to try and treat the boy under a compassionate use program with the experimental therapy.
In 2017, doctors collected hematopoietic stem cells from his bone marrow and blood from his mother. They broke open the walls of her white blood cells and used a centrifuge to spin out the mitochondria from the cytoplasmic sludge. Then they coaxed the boy’s stem cells to slurp up the mitochondria through a natural process called pinocytosis. Once the cells were augmented with the extra mitochondria, they were infused back into the child. He was the first, but five more children — three with Pearson and two with another deletion disease called Kearns-Sayre syndrome — followed.
Notably, the Sheba medical team decided to skip conditioning — a process that clears out a patient’s problematic hematopoietic stem cells to make way for modified ones, and a step that’s normally performed during many cell therapies. That decision was made, said Jacoby, because these children have trouble generating sufficient energy to overcome even mild infections and often suffer severe complications. Blasting out the tissues that produce their white blood cells was deemed too risky.
Because of that choice, and because the donor organelles came from the patients’ own mothers, the researchers had a much harder time measuring just how well the mitochondria augmentation worked. “We would love to be able to show the exact engraftment of each patient, but we just cannot track them [the donor mitochondria] well in the patients after transplant,” said Jacoby.
Instead, they measured the ratio of normal mitochondrial DNA to deleted DNA (which increased post-transplant), the total number of mitochondrial DNA molecules (which also increased), and the ability of cells to produce energy. (That improved too.) The data made Jacoby optimistic that the donor mitochondria survived and were contributing in their new cellular homes. “But these are all indirect measures,” he said.
in a group of infants with congenital heart defects. The small pilot study found that the mitochondria, when injected into the coronary artery, are rapidly taken up by injured heart cells where they boost energy production and other metabolic functions. The gains were significant enough that 80% of patients who received the treatment were able to be removed from a breathing-assistance machine, compared with 30% of patients who received standard of care, based on retrospective data.
“This is really powerful,” McCully said. “We’ve seen that it works in the heart, it works in the lungs, it works in the muscles. I really think the potential is essentially unlimited.”



ارسال به دوستان