Clinical trial shows promise of stem cells in offering safe, effective relief from arthritic knees
Stem cells collected from the patients own bone marrow holds great interest as a potential therapy for osteoarthritis of the knee (KOA) because of their ability to regenerate the damaged cartilage. The results were released today in STEM CELLS Translational Medicine (SCTM).
Stem cells collected from the patient"s own bone marrow holds great interest as a potential therapy for osteoarthritis of the knee (KOA) because of their ability to regenerate the damaged cartilage. The results were released today in STEM CELLS Translational Medicine (SCTM).
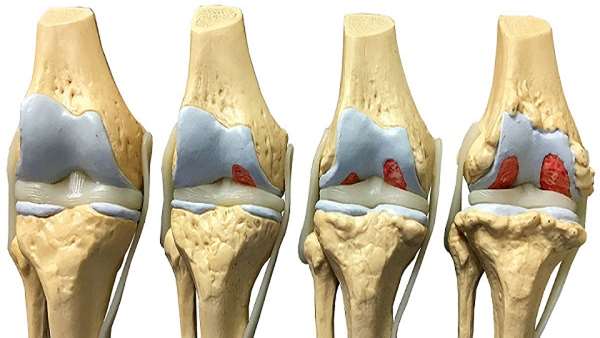
KOA is a common, debilitating disease of the aging population in which the cartilage wears away, resulting in bone wearing upon bone and subsequently causing great pain. In its end stages, joint replacement is currently the recommended treatment. In the first clinical trial of its kind to take place in Canada, researchers used mesenchymal stromal cells (MSCs), collected from the patient"s own bone marrow under local anesthesia, to treat KOA.
The study was conducted by a research team from the Arthritis Program at the Krembil Research Institute, University Health Network, Toronto, led by Sowmya Viswanathan, Ph.D., and Jaskarndip Chahal, M.D. "Our goal was to test for safety as well as to gain a better understanding of MSC dosing, mechanisms of action and donor selection," Dr. Viswanathan said.
It involved 12 patients, aged 45 to 65, with moderate to severe KOA. They were divided into three groups, with each group receiving a different dose of MSCs. (Each patient was injected with his or her own cells.) The researchers then followed the patients for the next 12 months, using analytical methods that included imaging, biomarkers, molecular fingerprinting and the patient"s own assessment of how he or she felt.
At the end of the 12-month period, the team noted significant improvements in the patients" pain levels and quality of life. The study also showed that the MSCs were safe at all the doses tested and that the higher the dose, the more effective the outcome.
Dr. Viswanathan said, "We also obtained novel insights into a potential anti-inflammatory mechanism of action of these cells in osteoarthritic knee joints. We noted that donor heterogeneity is an important factor, and our assembled panel of genes helps us identify cells which are potent in osteoarthritis. These are important findings which we hope to translate into a larger, powered clinical trial as part of our next steps."
"Furthermore," added Dr. Chahal, "we have been able to show that through an anti-inflammatory mechanism of action, such patients have an improvement in pain, function and quality of life. This sets the stage for the future of cell-based therapy and trials in Canada."
"This clinical pilot study advances the field of stem cell research for patients with arthritis, showing safety, and giving insights into potential therapy efficacy guidelines", said Anthony Atala, M.D., Editor-in-Chief of STEM CELLS Translational Medicine and director of the Wake Forest Institute for Regenerative Medicine. "We look forward to larger scale trial results."
Reference:DOI:10.1002/sctm.18-0183,https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.18-0183




ارسال به دوستان