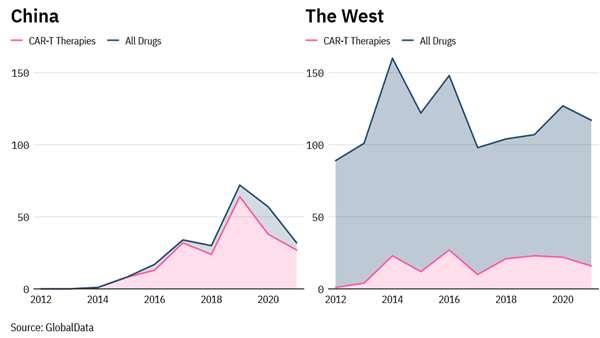
The development of CAR-T therapies in China appears to outpace research happening in the West, per an analysis by Pharmaceutical Technology. Between 2010 and 2014, over half of newly identified CAR-Ts were developed or co-developed by a US-based company. By 2021, that figure is expected to fall to less than one-third of new drugs.
The reason for this is not a drop-off in American focus on CAR-ts but instead an increase in new CAR-t therapies developed by Chinese companies. CAR-T therapies are a complex type of cell therapy where immune T cells are engineered to express chimeric antigen receptor (CAR) that binds to specific proteins on the tumor cells. Compared to other drugs, CAR-Ts require strict manufacturing and administration adherence, which makes their development resource-intensive and expensive.
Their development in different parts of the world was hampered by the need to make considerable upfront R&D, manufacturing, and scientific expertise. Most CAR-T therapies are developed in the US and commercial companies, but China is leading the field, with six of the ten companies involved in the development since 2010 being based in China. In the US, most CAR-T therapies are produced by pharmaceutical companies. In China, specialist biotechs are leading the field, and 80% of their drugs since 2010 are CAR-Ts. Legend Biotech bagged a US FDA approval for Carvykti (ciltacabtagene autoleucel), which co-developed with Janssen Biotech in multiple myeloma earlier this year. So far, China's regulatory agency has approved only two CAR-t therapies: Gilead's Yescarta and JW Therapeutics' Relma-cel. CAR-T therapies need a target antigen ubiquitously, and ideally exclusively, expressed in a tumor, which is the case with hematological cancers. Developing this modality in solid tumors has been and continues to be challenging.
https://www.pharmaceutical-technology.com/analysis/tracking-the-rise-of-car-ts-in-china-the-dawn-of-an-immunotherapy-superpower



ارسال به دوستان