Novel Type of Immune Cell Discovered in Type 1 Diabetes Patients
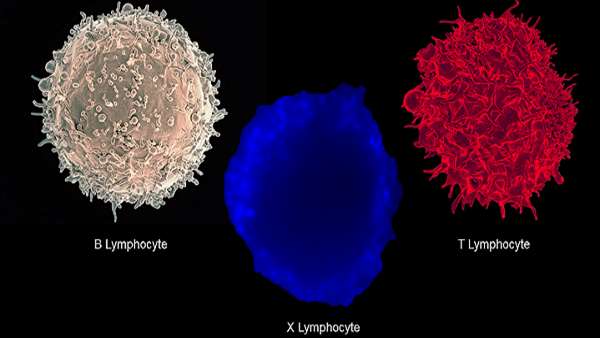
According to textbooks, cells of the adaptive immune system must be either B cells or T cells—they can’t be both, or anything in between.
According to textbooks, cells of the adaptive immune system must be either B cells or T cells—they can’t be both, or anything in between. But proving once again that nature is full of surprises, scientists have now discovered a novel type of lymphocyte in type 1 diabetes patients that combines characteristics of B cells and T cells. The researchers suggest that these hybrids could play an important role in the disease by encouraging the immune system to attack the body’s own insulin-producing cells, they report today (May 30) in Cell.
“The presence of a cell that expresses both B-cell receptors and T-cell receptors in and of itself is very novel,” remarks Jane Buckner, an immunologist and president of the Benaroya Research Institute, a Seattle-based nonprofit that conducts research on diseases of the immune system. However, more research is needed to establish its role in type 1 diabetes, adds Buckner, who wasn’t involved in the study.
The research team came across the rogue cells while they were looking for a specific type of B cell they had previously studied in the blood of type 1 diabetes patients. Using flow cytometry, they observed cells that present both B-cell and T-cell receptors on their cell surface, and upon further investigation, they revealed that the cells also express genes specific to both B and T cell lineages. These “dual expresser” (DE) cells were significantly more abundant in type 1 diabetes patients than in controls, explains Abdel Hamad, an immunologist at Johns Hopkins University School of Medicine and the senior author of the study.
The team made another unexpected discovery when analyzing the genomes of DE cells extracted from three unrelated people who all had type 1 diabetes. Embedded in the multiple genes that encode the cells’ B-cell receptor, Hamad’s group found a unique sequence that was present in the majority of DE cells. This was unusual, because that particular region of the B-cell receptor sequence is typically very diverse and differs starkly between the individual cells, Hamad explains. Notably, he and his colleagues could only find the sequence in DE cells from type 1 diabetes patients: they didn’t find it in DE cells from a healthy control subject, nor in a public database of 37 million B-cell receptor sequences from healthy participants.
These results made the team wonder if this sequence—and the peptide it produces—could play a role in driving type 1 diabetes. The basic mechanism of the disease is well characterized: T helper cells direct killer T cells to eliminate insulin-producing β cells in the pancreas, which ultimately deprives the body of insulin and the ability to regulate glucose, resulting in precariously high blood sugar levels. However, why T cells “see” insulin as a target is unclear.
A prevailing line of thought is that in type 1 diabetes, insulin is mistakenly presented to T cells by immune cells via particular human leukocyte antigen (HLA) molecules on their cell surfaces, which they typically use to present foreign pathogenic proteins as a means of training T cells to recognize them as invaders. One variant of this molecule, known as HLA-DQ8, is thought to present insulin to T cells in such a way that stimulates them to attack the hormone. (HLA-DQ8 is in fact overrepresented among type 1 diabetes patients).
The team surmised that the DE cells’ unique peptide could perhaps bind to the HLA-DQ8 molecule and thereby trigger T cells into action. To investigate this possibility, the team created a computational molecular simulation of the DE peptide and the HLA-DQ8 molecule, and found that indeed, the peptide binds to the molecule very strongly—around “ten thousand times more powerfully than the insulin peptide” itself, says Hamad. This means that the DE peptide can provoke a much stronger T-cell response against β cells than insulin itself, he adds.
In vitro experiments confirmed that the DE peptide bound tightly to the HLA-DQ8 molecule, and this stimulated T helper cells from type 1 diabetes patients to proliferate and to secrete proinflammatory cytokines. This specifically sparked activity among those T helper cells that had receptors with an affinity for insulin. Hamad and his team hypothesize that in vivo, the T helper cells then go on to direct certain killer T cells to eliminate β cells of the pancreas.
“We think [the DE cell peptide may play] a very major role during the initial phase of the disease,” Hamad says. He says he hopes the cells’ expression of the peptide could someday even be used as a biomarker to diagnose type 1 diabetes early on—something that is currently difficult to do. For him and his colleagues, the research raises many questions that need answering: whether the DE cells and their peptide are linked to a particular subset of patients or if they occur more generally, and how they arise to begin with. “That is a question that is keeping us awake at night: we don’t know exactly how they develop and where they develop,” he adds.
To Mark Peakman, an immunologist at King’s College London, the most surprising aspect of the study is the discovery of the new lymphocytes. “These cells, to my knowledge, have not been described by anybody in any other setting,” he notes. Until now, scientists have understood B and T cells as two lineages with a distinct development pattern: T cells grow up in the thymus, and B cells in the bone marrow and lymph nodes. “The textbooks will have to be rewritten if this study is true,” adds Peakman, who wasn’t involved in the new research.
However, he’s yet to be convinced about the cells’ role in driving type 1 diabetes: it’s not quite clear to him why the hybrid cells should produce a peptide that directs T cells to target insulin. The key element missing, he says, is a convincing link to insulin itself. The authors show that the T cells respond to the DE cell peptide, but they don’t show how insulin enters the picture. For instance, they don’t show whether the T cells are somehow primed by insulin or by a β cell presenting insulin first. “I’m struggling with this to see the connection to type 1 diabetes in mechanistic terms,” he says.
Buckner is similarly puzzled. Why this new type of cell should happen to express a peptide that mimics insulin and spurs T cells into attacking it is something that isn’t quite clear, she says. Especially with knowing so little about these cells, “I wasn’t able to quite pull that together as something that made a lot of sense to me,” she adds. For instance, do the cells normally play some beneficial role in fighting infections and its antigen expression simply goes awry in diabetes, or is the cell itself a freak occurrence that mostly occurs in diabetes to begin with? “I would like to understand if this cell—which they do see some of in healthy subjects—what this cell is, how frequent is this cell, is this cell unique to autoimmunity?”
Both Peakman and Buckner also raise some questions about the number and characteristics of patients studied in the research. “There are four or five patients that they focus on quite a lot in this paper,” several of whom have had diabetes for decades, Peakman notes. “Those are patients whose diabetes are as a process over, they have surely by that time not got any real level of β cell left, so why would these cells still be discoverable?” It might have been better to study patients close to diagnosis, or in children, he suggests.
But then again, autoimmunity is a field that does tend to throw up surprises, Peakman adds, noting that autoantibodies—those that target the body’s own proteins—weren’t discovered until the 1950s. “I think we have to be open to new findings and if it is true that there is a B-cell receptor on these cells that carries a [peptide] that’s important for type 1 diabetes, then time will tell and that will be replicated and validated in other studies.”



ارسال به دوستان