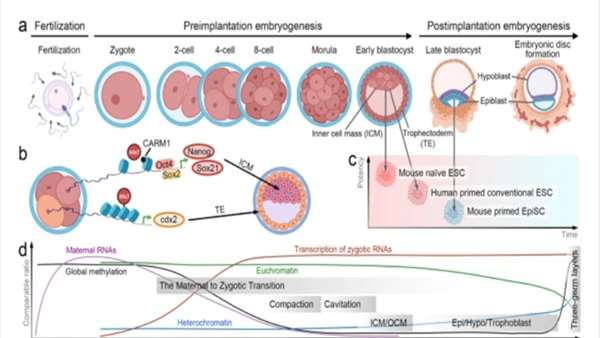
Each cell in the human body has a distinguishable fate. Pluripotent stem cells are challenged with a myriad of lineage differentiation options. Defects are more likely to be fatal to stem cells than to somatic cells due to the broad impact of the former on early development. In this review, we examine the events driving pluripotent stem cell fate and the underlying changes in gene expression during early development. In addition, we highlight the role played by the epitranscriptome in the regulation of gene expression that is necessary for each fate-related event.
Although somatic cells control chromatin accessibility to transcription machineries through their well-organized heterochromatin structure and epigenetic control, hPSCs accommodate the uncontrolled accessibility of transcriptional machineries to euchromatin throughout the nucleoplasm.
In particular, m6A is the most prevalent epitranscriptomic modification of mRNAs and is involved in the regulation of major signaling pathways in hPSCs. However, a number of studies have shown that mRNA modifications in addition to m6A were correlated with early developmental changes in animal models or modulated by molecular mechanisms during early developmental changes in PSCs. Therefore, future studies should be directed to investigating the complex epitranscriptomic gene regulatory system, which involves the mutual interaction of multiple types of RNA modifications, as well as the molecular mechanism and biological implications of certain types of RNA modifications.



ارسال به دوستان