Investigational New Drug (IND) Update
In this first IND update from CRISPR Medicine News, we look at three pre-clinical-stage CRISPR-engineered cell therapies that are under development for the treatment of sickle cell disease (SCD), beta-thalassemia, or acute lymphoblastic leukaemia (ALL).
In this first IND update from CRISPR Medicine News, we look at three pre-clinical-stage CRISPR-engineered cell therapies that are under development for the treatment of sickle cell disease (SCD),
beta-thalassemia, or acute lymphoblastic leukaemia (ALL).
CRISPR-based therapies are advancing rapidly with new clinical trials emerging all the time.
Before any new therapy can enter a clinical trial, its developers must obtain clearance from the relevant regulatory bodies.
In the US, the clinical trial sponsor, which is usually the company or institute developing the new therapy, must submit an Investigational New Drug (IND) application to the Food and Drug Administration (FDA) detailing the preliminary data gathered from cellular models and animal studies. IND approval must be obtained before any clinical trial can take place. In Europe, preliminary safety data is included with a clinical trial application (CTA) to the European Medicines Agency (EMA).
Once approval is obtained for clinical testing by the relevant regulatory body i.e. FDA, EMA or otherwise, the programme may enter clinical development in that territory.
In this first IND update from CRISPR Medicine News, we look at three pre-clinical-stage CRISPR-engineered cell therapies that have obtained IND designation for the treatment of sickle cell disease (SCD), beta-thalassemia, or acute lymphoblastic leukaemia (ALL).
FT819: An Off-The-Shelf CAR T-Cell Therapy for Acute Lymphoblastic Leukaemia
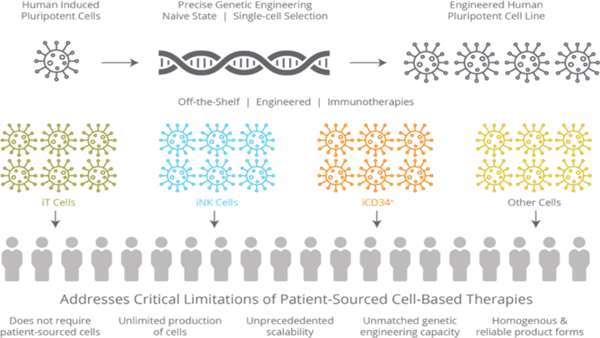
Californian biopharmaceutical company Fate Therapeutics has a pipeline of induced pluripotent stem cell (iSPC)-derived cellular immunotherapies for cancer and immune disorders. One of these is FT819, an off-the-shelf CAR T-cell cancer immunotherapy candidate for the treatment of acute lymphoblastic leukaemia (ALL). FT819 is CRISPR-engineered to express a novel 1XX CAR that targets the CD19 B cell-specific cell surface antigen that is expressed in all B cell lineage malignancies.
Fate Therapeutics announced that it had obtained IND clearance from the FDA in July of this year, allowing it to proceed with the clinical development of FT819 for treatment of CD19+ cancers. FT819 is the first-ever CAR T-cell therapy to be derived from a clonal master iPSC line engineered with several first-of-kind features that are designed to improve the safety and efficacy of CAR T-cell therapy.
In FT819, the 1XX CAR is inserted into the T cell receptor alpha constant (TRAC) locus, which disrupts the native T cell receptor (TCR) locus while simultaneously placing the CAR under the regulatory control of the endogenous TCR promoter. Inserting a CAR at this location has previously been demonstrated to improve T cell function and potency in mouse models of ALL.
At last year's American Hemotology Association Annual Meeting, Fate Therapeutics presented encouraging in vivo preclinical data demonstrating that FT819 exhibits durable tumour control and extended survival in xenograft mouse models of ALL.
This was our first update about pre-clinical stage therapies based on gene editing. If you want to learn more about the clinical development path, you can check out our earlier explainer piece that covers the path from IND filing right through to Phase IV (post-market surveillance).
https://crisprmedicinenews.com/news/investigational-new-drug-ind-update/



ارسال به دوستان