In their DNA: Rotator cuff stem cells more likely to develop into fat cells
Why are fat deposits more likely to occur after tears of the shoulders rotator cuff, compared to other types of muscle injuries? An increased propensity of stem cells within with rotator cuff muscles to develop into fat cells may explain the difference, reports a study in the February 6, 2019 issue of The Journal of Bone & Joint Surgery.
Why are fat deposits more likely to occur after tears of the shoulder"s rotator cuff, compared to other types of muscle injuries? An increased propensity of stem cells within with rotator cuff muscles to develop into fat cells may explain the difference, reports a study in the February 6, 2019 issue of The Journal of Bone & Joint Surgery.
"Satellite" stem cells in the rotator cuff are more likely to develop into fat cells and less likely to develop into muscle cells, compared to calf muscle satellite cells, according to the experimental study by Christopher L. Mendias, Ph.D., ATC, and colleagues of the University of Michigan Medical School, Ann Arbor, and the Hospital for Special Surgery, New York. The researchers write, "There appears to be a cellular and genetic basis behind the generally poor rates of rotator cuff muscle healing."
"Satellite Cells" May Form More Fat Than Muscle after Rotator Cuff Tears
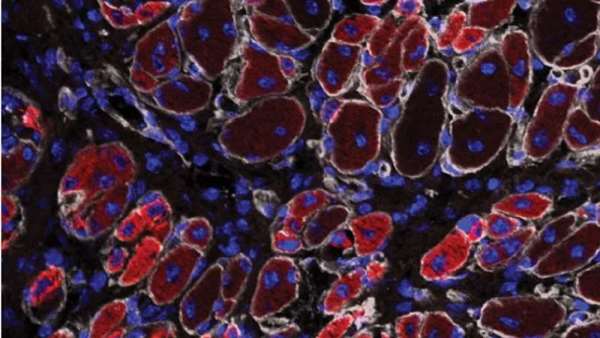
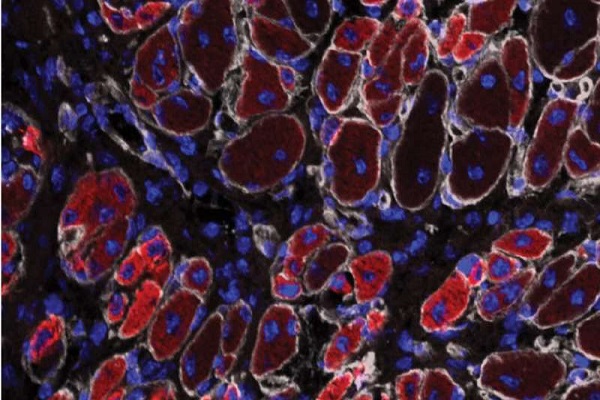
The researchers performed a series of experiments using muscle cells from mice to evaluate the characteristics of a type of stem cells called satellite cells. Stem cells are specialized cells with the potential to develop into different types of cells. Satellite cells, located between muscle fibers, play an essential role in repair after muscle injuries.
Tears of the shoulder rotator cuff are a common problem. Especially in chronic tears, deposits of fat often develop, contributing to weakening and atrophy of the rotator cuff muscles. This fatty infiltration can continue even after successful rotator cuff repair surgery.
Dr. Mendias and colleagues created cultures of satellite cells isolated from mouse rotator cuff and calf muscles. "Clinically, we know that the rotator cuff is one of the most difficult muscle groups to rehabilitate after injury, and this is thought to occur due to the extensive fat that accumulates in the muscle in patients with chronic tears," says lead author Manuel Schubert, MD, MS, chief resident in orthopaedic surgery at the University of Michigan. "We thought there might be a genetic basis to explain why the rotator cuff accumulates fat after injury, and the specialized transgenic model we used in this study allowed us to precisely test this."
Compared to the calf muscle satellite cells, satellite cells from the rotator cuff developed into 23 percent fewer muscle cells, and they showed an 87 percent decrease in a "marker" for muscle formation. The rotator cuff satellite cells also had a four- to 65-fold increase in markers of genes involved in fat cell generation (adipogenesis).
DNA-level (epigenetic) studies identified hundreds of differences in gene activation between satellite cells from rotator cuff versus calf muscles. The affected genes were involved in pathways related to fat metabolism and adipogenesis, suggesting the muscle stem cells from the rotator cuff are programmed to more easily become fat cells.
Building on previous research, the new study shows increased "adipogenic differentiation capacity" of rotator cuff satellite cells. Increased potential to develop into fat cells—and decreased potential to develop into muscle cells—may be an important explanation for the high rate of fatty infiltration in muscles of patients with chronic rotator cuff tears, even after rotator cuff surgery.
The study also has potential therapeutic findings. "Satellite cells can be isolated from other muscle groups with relative ease." says Dr. Mendias, an Associate Scientist at the Hospital for Special Surgery and an Adjunct Associate Professor at the University of Michigan. "While further studies are necessary, it is possible that a patient"s own stem cells from a muscle that heals well, like the calf, could be transplanted to the rotator cuff muscle at the time of surgical repair. These transplanted cells might be better able to regenerate the chronically damaged muscle than the resident stem cells."
Reference:DOI:10.2106/JBJS.18.00509,https://www.eurekalert.org/pub_releases/2019-02/journals.lww.com/jbjsjournal/Fulltext/2019/02060/Reduced_Myogenic_and_Increased_Adipogenic.4.aspx



ارسال به دوستان