Immunotherapy has revolutionized many treatments in hematologic malignancies over the past decade, from monoclonal antibodies to check point inhibitors and specific T cell engagers. Much progress has occurred in that aspect. Now the task is to figure out where each therapy fits the best for the patient. Applying this data in the real world is challenging.
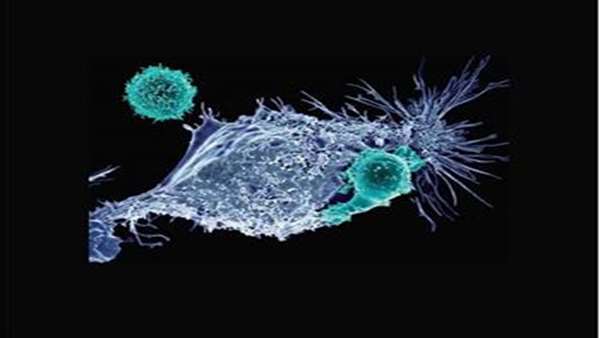
In 2018, blinatumomab (Blincyto) was approved for pediatric acute lymphocytic leukemia (ALL). This CD19-targeted BiTE (bispecific T-cell engager) therapy is now approved for relapsed or refractory B-cell ALL in adults. Tisa-cel was the first CAR-T product approved by the FDA. Cytokine release syndrome (CRS), a common complication seen in patients receiving CAR T therapy, is noted in most CAR T trials. Some products have higher CRS toxicities than others. The approval of the third CAR T, liso-cel, was based on the TRANSCEND-NHL-001 trial. The objective response rate in this trial was 73%, with a 53% complete response rate. Brexa-cel received approval for adult relapsed or refractory B-cell precursor ALL after investigators published results from ZUMA-3. The FDA approved the Cilta-cel regimen investigated in the CARTITUDE-1 trial in February 2022. CAR T cannot be used for AML or solid tumors, as there is limited infiltration in solid tumors and antigen escape. Researchers are investigating allogeneic CAR T products where T cells are collected from donors and infused into patients. There are also trials looking at CAR natural killer (NK) cells instead of T cells. In the years to come, CAR T will likely be combined with checkpoint inhibitors and other immunotherapies in different solid tumors, as well as a range of hematologic malignancies to determine the best outcomes.
https://www.targetedonc.com/view/advances-in-car-t-cell-therapy-in-hematologic-malignancies



ارسال به دوستان