A new gene therapy transplantation technique could improve treatment of neurodegenerative diseases
Immune cells defending the central nervous system (the so-called microglia) have a key role in many neurodegenerative diseases. A study published today in Science Advances shows for the first time the efficacy of a new gene therapy transplantation technique which aims at repopulating the brain with new, genetically engineered immune cells.
Immune cells defending the central nervous system (the so-called microglia) have a key role in many neurodegenerative diseases. A study published today in Science Advances shows for the first time the efficacy of a new gene therapy transplantation technique which aims at repopulating the brain with new, genetically engineered immune cells. Such cells are generated by progenitors which are injected directly into brain ventricles - that makes their therapeutic action quicker. The research was performed at San Raffaele Telethon Institute for Gene Therapy (SR-Tiget) with the collaboration of the Dana-Farber/Boston Childrens Cancer and Blood Disorders Center. The scientists have been guided by Alessandra Biffi, supervisor of a research unit at SR-Tiget and director of the Gene therapy program at Dana-Farber/Boston Childrens. The technique has been tested on an experimental model for a metabolic disease and might have future therapeutic applications for other neurodegenerative diseases in which microglia cells play a role. The study was supported also by the European Commission through an ERC grant won by Alessandra Biffi.
This study has its roots in Alessandra Biffis long-standing interest in lysosomial storage diseases (LSDs), a heterogeneous group of hereditary metabolic diseases caused by a genetic mutation preventing cells from producing enzymes which are fundamental for their metabolism. Patients with LSDs have severe damages to different organs and tissues, including the central nervous system. The standard therapeutic approach - which is often the only one at hand - consists in the injection in the blood stream of the missing enzyme. That, though, is ineffective in case the patient has already shown neurological problems - in fact, the blood-brain barrier prevents the enzyme from reaching the brain, where neurodegeneration continues. That is why a few years ago Alessandra Biffi, working together with Luigi Naldini (director of SR-Tiget), tried cell and gene therapies approaches. In an ongoing clinical trial performed at SR-Tiget to treat metachromatic leukodystrophy (a neurodegenerative LSD), blood stem cells are extracted from the patients bone marrow, genetically engineered so as they regain the ability to produce the missing enzyme, and then injected back into the bloodstream. Being able to cross the blood-brain barrier, the cells reach the brain and begin their therapeutic action.
Timing, though, is a key element. Gene therapies administered through the blood are effective only if they act in advance. Cells, in fact, need time to reach the brain and engraft into the nervous tissue. That is why at the moment only patients who are still asymptomatic can be treated.
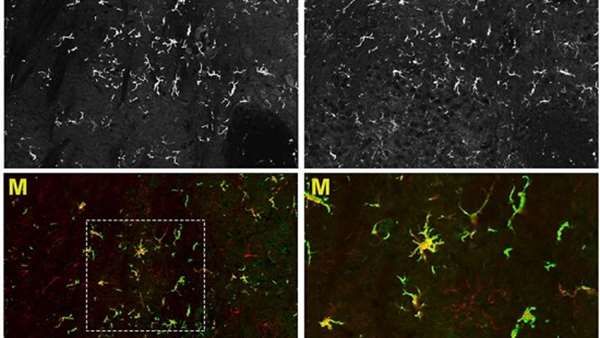
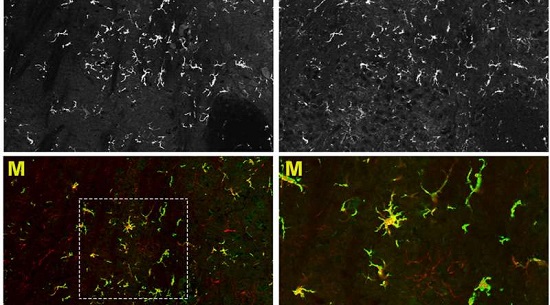
The new technique designed by Alessandra Biffis team and discussed on todays issue of Science Advances could change everything. "Transplanting stem cells into brain ventricles quickens the engraftment process and in the future could be a viable therapeutic option for patients showing the first symptoms, too" says Alessandra Biffi. But the research results extend beyond the timing question. "Our data shows for the first time that transplanted blood stem cells, once they reach the brain, are able to differentiate into microglia-like cells. These cells remain exclusively in the central nervous system". This means that with such technique we can quickly generate a population of genetically engineered cells exclusively in the ill nervous system. From their position, these cells can release therapeutic molecules, helping the tissue to heal and regenerate, and might have potential application in a variety of neurodegenerative diseases.
The study was supported by Fondazione Telethon and the European Commission - ERC Consolidator Grant "HSCSFORLSDBRAIN".
Dana-Farber/Boston Childrens Cancer and Blood Disorders Center brings together two internationally known research and teaching institutions that have provided comprehensive care for pediatric oncology and hematology patients since 1947. The Harvard Medical School affiliates share a clinical staff that delivers inpatient care and surgery at Boston Childrens Hospital, outpatient oncology care at Dana-Farber Cancer Institute and outpatient blood disorders care at Boston Childrens.
Reference: http://advances.sciencemag.org/content/3/12/e1701211



ارسال به دوستان