According to the report of Public Relations and International Affairs of the Stem Cell Science and Technology Development council, in a interviewer with Dr. Ensieh Hajizadeh Saffar, director of the Royan ATMP Technology Development Center, regarding receiving GMP certification from the Food and Drug Administration for KIMIACELL production, said the Royan ATMP Technology Development Center was put into operation in May 2018. The mission of this center is to create a safe and efficient bridge between the laboratory and preclinical stages of the development of products based on regenerative medicine to reach the stage of treatment and industrial production of the product on a large scale. Therefore, this center has created a strong scientific infrastructure for the development of products based on regenerative medicine after selecting the product among the qualified research projects in Royan Research Institute, which produces and controls the quality of cell products and quality assurance of related products in GMP space according to international standards.
She added: "Currently, the development of several products in the field of cell therapy, gene therapy and tissue engineering for the treatment of various incurable diseases is on its agenda. Development of advanced cell-based products in the field of orthopedic, skin, degenerative diseases, autoimmune diseases and eye, neurologic and metabolic disorders, setting up a reference laboratory for quality control of cells and related products as a partner laboratory of the Food and Drug Administration and also developing accurate and reliable standards for production and quality assurance of products based on regenerative medicine is one of the strategic goals of this center.
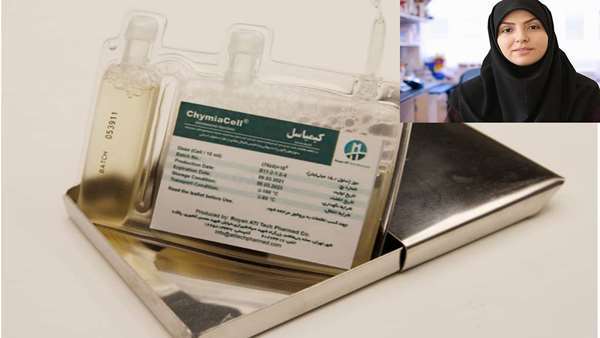
Rheumatoid arthritis is the most common systemic inflammatory disease and is considered a chronic autoimmune disease. Its global prevalence is reported to be about one percent, and chronic disability is common and significant. Since 2016, a solution for the treatment of patients with rheumatoid arthritis has been formed at the Royan ATMP Technology Development Center, and the researchers' efforts to produce a KIMIACELL product in 2021 have led to the observance of all international standards. In the first step, it succeeded in receiving the GMP certificate from the Food and Drug Administration of Iran. Doctors will be experts to carefully evaluate the safety and effectiveness of this product. It should be mentioned that this product and several other products in the field of cells and tissues have reached the stage of industrial production under the joint investment of Lotus Parsian Foundation and Royan Research Institute.
She then stated: "The KIMIACELL product contains clonal stromal mesenchymal cells isolated from the bone marrow of a healthy and qualified donor, which is produced, prepared and packaged in completely aseptic and GMP conditions." These cells are directly and indirectly responsible for a variety of consequences that occur in the body, such as participating in the repair of damaged tissues and anti-inflammatory effects. Restorative properties and their differentiation into other cell lines (potency and plasticity) and secretion of anti-inflammatory vesicles have made its the safest and most accessible cells for the treatment or at least mitigation of complications of various diseases in regenerative medicine. Safety, identification, efficacy, purity and performance tests of the final KIMIACELL product have been confirmed by various quality control tests.
Finally, he said: "Among the characteristics of the KIMIACELL product are the following: immunomodulatory properties, the ability to reduce the inflammatory response by suppressing lymphocytes and thus reducing the secretion of pro-inflammatory cytokines, the ability to repair damaged tissues such as bone and cartilage in two ways Direct (differentiation into osteoblasts and condocytes) and indirect (secretion of paracrine molecules), angiogenic properties by secreting factors involved in the angiogenesis process, including VEGF, resulting in the delivery of oxygen and nutrients to cells in the affected area, including KIMIACELL applications for Treatment for patients with rheumatoid arthritis, chronic low back pain due to disc destruction, as well as osteoarthritis of the knee.



ارسال به دوستان