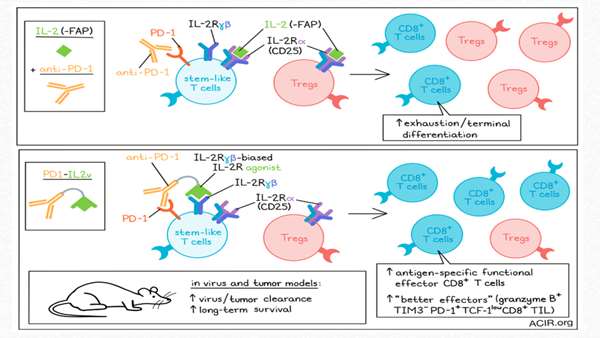
The researchers engineered an immunocytokine, PD1-IL2v, that induces stronger and more exclusive expansion of better effector cells
IL-2 can induce the differentiation of stem-like T cells into CD8+ T cell populations with better effector functions in chronic infection. However, systemic treatment with IL-2 induces adverse effects, such as the expansion of Tregs. To overcome this problem, Deak, Nicolini, Hashimoto, and Karagianni engineered an immunocytokine, PD1-IL2v, that induces stronger and more exclusive expansion of better effector cells. This therapy was tested in chronic infection and cancer models, and the results were recently published in Nature.
The researchers designed PD1-IL2v to provide IL-2R agonism preferentially to PD-1+ tumor- or virus-reactive T cells, linking a PD-1-binding antibody to an IL-2 receptor β- and γ-chain-biased agonist, thus blocking the PD-1 pathway. To assess whether PD1-IL2v would have stimulating effects on Tregs, binding competition and suppression assays with Tregs and conventional CD4+ T (Tconv) cells were performed. PD1-IL2v bound preferentially to the Tconv, which had higher levels of PD-1 than Tregs. The binding of PD1-IL2v to Tconv helped these cells overcome Treg mediated suppression in a dose-dependent manner.
T lymphocytes that express an αβ T cell receptor (TCR), as well as a co-receptor CD4 or CD8, are present in the peripheral blood, lymph nodes, and tissues (such as the skin), and are considered conventional T cells.
The researchers found that PD1-IL2v could also induce GM-CSF and granzyme B secretion in PD-1+CD4+ T cells in a dose-dependent manner.
GM-CSF is a growth factor that increases the number of circulating white blood cells and enhances neutrophil and monocyte function.
Granzyme B is a serine protease that initiates a series of signals within the target cell and induces programmed cell death. Granzyme B causes the disintegration of structures such as the mitochondrial membrane and changes its permeability. On the other hand, by stimulating the secretion of other types of proteases, it induces DNA destruction. The pro-apoptotic function of granzyme B underlies the ability of cytotoxic immune cells, such as cytotoxic T lymphocytes (CTLs) and natural killer cells (NK) to destroy target cells infected with viruses and tumors.
The combination of anti-PD-L1 and PD1-IL2v was superior to anti-PD-L1 monotherapy in increasing the number of LCMV-specific CD8+ T cells in various tissues. These specific T cells had a polyfunctional signature (producing IFNγ, TNFα, and/or IL-2) and therapy-induced changes in the expression of phenotypic markers. increased expression of Cd28, various cytokine receptors, chemokines related to T cell migration, adhesion molecules, and molecules related to egress from lymphoid tissues are features of functional effector CD8+ T cells, while exhausted T cell markers and transcription factors such as Tox and Pdcd1 were downregulated. PD1-IL2v monotherapy also induced expansion of LCMV-specific CD8+ T cells, but the combination treatment increased numbers more and resulted in more marked phenotypic changes.
To assess antitumor effects, mice bearing subcutaneous Panc02-H7Fluc tumors were then treated with PD1-IL2v, high-dose IL-2, or either monotherapy combined with pembrolizumab (anti-PD-1). Survival and tumor growth reduction were more pronounced in mice treated with PD1-IL2v monotherapy than those treated with the combination of high-dose IL-2 and pembrolizumab. The combination of PD1-IL2v and pembrolizumab did not impair the efficacy, but also did not induce additional benefit.



ارسال به دوستان