Generation of a Motor Nerve Organoid with Human Stem Cell-Derived Neurons
During development, axons spontaneously assemble into a fascicle to form nerves and tracts in the nervous system as they extend within a spatially constrained path. However, understanding of the axonal fascicle has been hampered by lack of an in vitro model system.
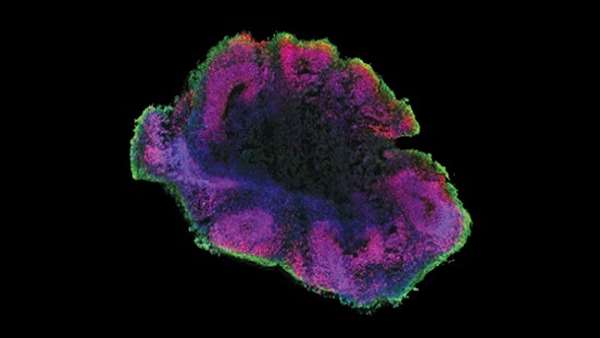
During development, axons spontaneously assemble into a fascicle to form nerves and tracts in the nervous system as they extend within a spatially constrained path. However, understanding of the axonal fascicle has been hampered by lack of an in vitro model system. Here, we report generation of a nerve organoid composed of a robust fascicle of axons extended from a spheroid of human stem cell-derived motor neurons within our custom-designed microdevice. The device is equipped with a narrow channel providing a microenvironment that facilitates the growing axons to spontaneously assemble into a unidirectional fascicle. The fascicle was specifically made with axons. We found that it was electrically active and elastic and could serve as a model to evaluate degeneration of axons in vitro. This nerve organoid model should facilitate future studies on the development of the axonal fascicle and drug screening for diseases affecting axon fascicles.
During development, neurons spontaneously assemble to build organized structures and functional circuits in various parts of the nervous system. A fascicle of axons is one of the major structural motifs often observed in the nervous system, e.g., the peripheral nerves, corticospinal tract, and corpus callosum (Ahsan et al., 2007, Stewart, 2003). Previous studies have shown that an axonal fascicle is formed as growing axons follow and gather along the preceding “pioneer axon” through axo-axonal interactions in vivo; however, further details of the mechanism of this process are yet to be clarified (Raper and Mason, 2010). Importantly, axon fascicles are often damaged by neurodegenerative diseases (Ito et al., 2016). For instance, previous studies suggest that amyotrophic lateral sclerosis disrupts axonal maintenance mechanisms, including the protein transportation system, thereby causing specific elimination of motor neurons (Alami et al., 2014, Bilsland et al., 2010, LaMonte et al., 2002, Morfini et al., 2009). Although numerous insights into the developmental and pathophysiological mechanisms have been derived from studies on single axons in dissociated 2D culture, we have little insight into fascicles of axons due to the lack of in vitro culture systems.
Human stem cell-derived neurons could provide a promising platform in drug discovery and the development of therapeutics for devastating neurological disorders, and they have been extensively studied for understanding neuronal development, functions, and diseases (Sances et al., 2016, Shi et al., 2012, Sterneckert et al., 2014). Since it is increasingly apparent that proper multi-cellular organization and microenvironment are critical for physiological modeling of development and diseases of the nervous system, in vitro three-dimensional brain “organoid” culture systems have been proposed to provide improved systems that more accurately represent multi-cellular structures (Lancaster et al., 2013, Sasai, 2013). The emerging technology currently relies on cell-intrinsic, self-assembly mechanisms to form tissue morphology, and few extrinsic environmental factors are considered. By developing methods that can form organoids representing specific parts of the nervous system, we would be able to model development and diseases of specific neuronal structures and circuitry.
In this study, we developed a method for the formation of a nerve organoid composed of a fascicle of axons extended from a spheroid of human stem cell-derived motor neurons. By providing a spatially confined environment in a microchannel, axons spontaneously assembled into a unidirectional fascicle within the microchannel. The resultant fascicle of axons can be subjected to various examinations, including morphological, electrical, and physical analyses. This nerve organoid model mimics development and dysfunction of a human motor nerve, thus potentially serving as a platform for high-throughput screening.



ارسال به دوستان