Fate Therapeutics Announces Off-the-Shelf CAR T-cell Cancer Immunotherapy to be Featured at 2018 AACR Annual Meeting Press Program
Fate Therapeutics a clinical-stage biopharmaceutical company dedicated to the development of programmed cellular immunotherapies for cancer and immune disorders, announced today that the Company is presenting new preclinical data on FT819, its off-the-shelf CAR T-cell product candidate, at the American Association for Cancer Research (AACR) Annual Meeting being held from April 14-18, 2018 in Chicago, Illinois.
Fate Therapeutics a clinical-stage biopharmaceutical company dedicated to the development of programmed cellular immunotherapies for cancer and immune disorders, announced today that the Company is presenting new preclinical data on FT819, its off-the-shelf CAR T-cell product candidate, at the American Association for Cancer Research (AACR) Annual Meeting being held from April 14-18, 2018 in Chicago, Illinois.
The presentation of FT819 was accepted by AACR as a late-breaking abstract, and was subsequently selected by AACR to be featured at the AACR Annual Meeting press program being held today at 8:30 a.m. CT.
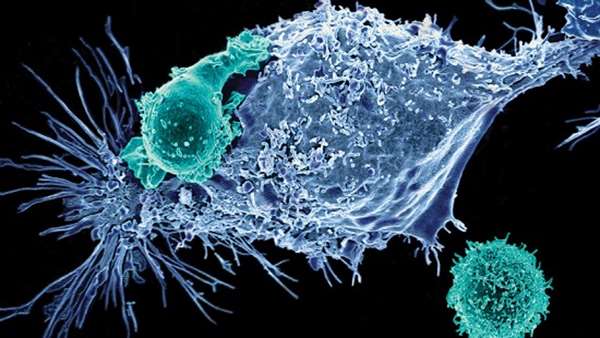
FT819 is an off-the-shelf CAR T-cell product candidate produced from a master induced pluripotent stem cell (iPSC) line. FT819 has two targeting receptors, a chimeric antigen receptor (CAR) targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other proven cancer therapies, such as tumor antigen-targeting monoclonal antibody (mAb)-based treatments, to overcome antigen escape. Fate Therapeutics is developing FT819 as part of a research collaboration being led by Michel Sadelain, M.D., Ph.D., Director, Center for Cell Engineering, Memorial Sloan Kettering Cancer Center.
In preclinical studies, FT819 exhibited an efficient cytotoxic T-cell response in vitro when challenged with CD19-positive tumor cells, displaying robust production of effector cytokines, including INF-gamma and TNF-alpha, and cytolytic proteins, including perforin and granzyme B. The product candidate’s activity was also found to be target-specific in vitro, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells. Additionally, when combined with a mAb-based treatment targeting CD20, FT819 was shown to elicit antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro against CD19-negative, CD20-positive tumor cells through CD16 engagement.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert CAR19 into the T-cell receptor α constant (TRAC) locus for enhanced safety and potency and to completely eliminate T-cell receptor (TCR) expression. The line serves as a renewable source for consistently and repeatedly manufacturing homogeneous cell products in quantities that support the treatment of many thousands of patients in an off-the-shelf manner. This approach eliminates the need to create a personalized therapy from a patient’s own cells, enables mass production at scale and significantly reduces the cost of, and time to, patient treatment.
The data is also being presented by the Company in a poster session.



ارسال به دوستان